Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Energy & Fuels
Flowback-produced water (FP) is a waste fluid associated with hydraulic fracturing in unconventional oil and gas development (UOG). Initially, FP reflects the composition of the hydraulic fracturing fluid, which is referred as flowback water (FBW). After the initial months of well production, the waste fluid is predominantly representative of the formation and is known as produced water (PW).
- produced water
- hydraulic fracturing
1. Technologies Utilized in Produced Water Treament
The major concern in treating FP for reuse, apart from the cost of treatment, is the removal of pertinent constituents (see Table 1) that can negatively affect the production of a given oil/gas well. For example, elevated levels Sr, Ca, Mg and Ba can contribute to the formation of insoluble scales in production tubing, which can attenuate production rates [48]. Elevated levels of sulfate can also contribute to scaling, as well as provide a substrate for sulfate-reducing bacteria (SRB) to proliferate. Ultimately, this could lead to the corrosion of tubing and, as a consequence, environmental contamination along with the clogging of the wellbore, the degradation of hydrocarbons and the souring of natural gas [16,17,25,32,49]. Additionally, significant concentrations of B and Fe (>10 mg/L) limit effectiveness of cross-linkers polymerization in fracturing fluid [16,50]. Lastly, elevated values of TOC, Na, Ca, Fe and phosphate reduce the viscosity of gel-based fracturing fluids [48], which can have negative implications for production well stimulation.
The biogeochemical complexity of produced water requires the implementation of multiple treatment modalities to effectively remove all the contaminants from microorganisms and heavy metals to organic particulates and NORMs. The most widely utilized procedures can be categorized as such: chemical oxidation, adsorption, membrane filtration, electrocoagulation and distillations:
-
Chemical oxidation facilitates the flocculation of volatile and semi-volatile organics, the precipitation of inorganic compounds, and the eradication of bacteria. Additionally, the use of oxidizing agents leads to the volatilization and remediation of undesirable odors and colors, respectively. The oxidizing agents most commonly used in FP treatment include ozone, hydrogen peroxide, chlorinated compounds and permanganate [4]. Advanced oxidation processes (AOPs) comprise a set of chemical treatments that remove organic matter by reaction and subsequent degradation with a hydroxyl (OH) group. Furthermore, AOPs are thought to be environmentally sustainable for chemical oxygen demand (COD) degradation [13]. Recent advances in this technology involve the addition of nanoparticles to enhance the removal of major organics from fracking wastewater [51].
Table 1. Inorganic constituents and other parameters of fracturing waste waters from Bakken Shale and Permian Basin, the regulated concentration ranges for reuse in well stimulation [15] and in agricultural and consumption use [52,53]. * represents the reported average of three measurements in the study.
| Bakken Shale Range (mg/L) [13,40,54,55,56,57] |
Permian Basin Range (mg/L) [6,58,59] |
Well Stimulation (mg/L) [15] |
Agricultural Use (mg/L) (EPA) |
Drinking Water (mg/L) (FAO & EPA) |
|
|---|---|---|---|---|---|
| METAL | |||||
| Magnesium (Mg) | 1530–3790 | 1630–1950 | 2000 | ||
| Iron (Fe) | 0.70–30.20 | 11 | 10.00 | 5.00 | 0.30 |
| Manganese (Mn) | 5.20–17.20 | 11.00–53.00 | 0.20 | 0.05 | |
| Aluminium (Al) | <LOQ–8.30 | 5.00 | 0.05–0.20 | ||
| Calcium (Ca) | 13,140–41,160 | 10,000–15,000 | 2000 | ||
| Sodium (Na) | 89,100–189,000 | 48,000–54,000 | 69.00 | ||
| Potassium (K) | 3510–9530 | 570–1100 | |||
| Barium (Ba) | 6.40–26.30 | 0.00–16.00 | 20.00 | 2.00 | |
| Strontium (Sr) | 709–2450 | 730.0–820.0 | |||
| Cobalt (Co) | 0.030–0.20 | N/A | 0.050 | ||
| Nickel (Ni) | <LOQ–3.80 | 0.020 | 0.20 | 0.07 | |
| Lithium (Li) | 34.50–89.70 | 18.80 | 2.50 | ||
| Chromium (Cr) | 0.10 | 0.10 | |||
| Radium 226 (Ra) | 527.1–1211 pCi/L | 5.000 pCi/L | |||
| Uranium (U) | 30.00 µg/L | ||||
| Copper (Cu) | 4.60–16.90 | 0.20 | 1.00 | ||
| Zinc (Zn) | 2.50–10.10 | 2.00 | 5.00 | ||
| Arsenic (As) | 1.1 | 0.10 | 0.01 | ||
| Beryllium (Be) | 0.10 | 0.004 | |||
| Lead (Pb) | 0.00–3.50 | 5.00 | 0.015 | ||
| Silver (Ag) | 0.10 | ||||
| Molybdenum (Mo) | 0.01 | ||||
| Cadmium (Cd) | 0.001–0.031 | 0.01 | 0.005 | ||
| Vanadium (V) | 0.60–1.00 | 0.10 | |||
| Thallium (Tl) | 0.00–0.20 | 0.002 | |||
| Antimony (Sb) | 0.006 | ||||
| Rubidium (Rb) | 0.30–12.90 | ||||
| Mercury (Hg) | 0.002 | ||||
| NON-METAL | |||||
| Chloride (Cl−) | 21,728–136,220 | 111,000–138,000 | 30,000–50,000 | 92.00 | 250.0 |
| Bromide (Br−) | 91.6–558 | 1370–1650 | |||
| Silicon (Si) | 32 | 35.00 | |||
| Fluoride (F−) | 1.00 | 4.00 | |||
| Boron (B) | 25.0–260.1 | 10.00 | 0.70 | ||
| Selenium (Se) | 0.10–1.00 | 0.02 | 0.05 | ||
| POLYATOMIC IONS | |||||
| Sulfate SO42−) | 0.000–293.0 | 515–743 | 500 | 250 | |
| Bicarbonate (HCO3−) | 35.00–856.0 | 92–160 | 300 | 91.50 | |
| Nitrite (NO2−) | 1.00 | ||||
| Nitrate (NO3−) | 5.000 | 10.00 | |||
| Phosphate (PO43) | 584 * | ||||
| Ammonium (NH4+) | 44.8–2520 | 655 | |||
| Cyanide (CN−) | 0.200 | ||||
| OTHER PARAMETERS | |||||
| pH | 4.1–7.2 | 7.30 | 6.0–8.0 | 6.5–8.4 | 6.5–8.5 |
| TDS | 128,300–388,600 | 174,213–212,984 | 450 | 500 | |
| TSS | 7040 * | 6850–21,820 | 500 | ||
| Total nitrogen | |||||
| TOC | 311 * | 86.25–184.21 | |||
| Alkalinity (CaCO3) | 0–562.8 | 2345 | |||
| Turbidity (NTU) | 13 | 53.4 | |||
| DOC | 80 * | 63.45–145.71 | |||
| Conductivity (mS/cm) | 201.2 | ||||
| Nonvolatile dissolved organic carbon (NVDOC) | 1.13–3.31 | ||||
| Total Hardness(mg/L CaCO3) | 31,000–59,000 | ||||
| Chemical Oxygen demand (COD) | 20,000–79,000 | ||||
- 2.
-
Adsorption is applied for the sequestration of organics and metal contaminants. However, it is more of a polishing step for other preceding treatment modalities instead of being a sole separation technique on its own. It is important to note that the adsorption efficiency of various media is mediated by salinity. Activated carbon media are effective for organic contaminants, whereas, activated zeolite is an effective adsorbent for the removal of scaling ions such as Ca2+ and Mg2+ [60] that are generally present in elevated concentrations in FP (see Table 1). Other possible absorbents include alumina and organoclays [4]. In recent studies, Sun et al. achieved the removal of several metal pollutants; for instance, Cu(ll), As(V), Cr (Vl), Cr(ll) and Zn(ll) on Fe-impregnated biochar, a carbon-rich fine-grained pyrolysis residue [61].
- 3.
-
Membrane filtration consists of the separation of a fluid from dissolved substances by a porous surface. This includes reverse osmosis (RO), microfiltration (MF), nanofiltration (NF), ultrafiltration (UF) and forward osmosis (FO). RO removes solids by the application of hydraulic pressure to move water molecules through a semi-permeable membrane; MF allows the physical separation of suspended solids and turbidity depletion via the retention of particles larger than the micropores in the membranes. UF reduces odor, organic matter and color with pore membranes on the order of microns. NF offers selective particle rejection based on size and charge, which lessens multivalent ions, and FO lowers TDS in high-saline brines, benefiting from osmotic pressure and transporting water molecules through a semipermeable membrane from the less-concentrated feed to the highly concentrated solution [62]. Some modalities could be applied as treatment technologies on their own, such as MF and UF; others are steps in a more complex separation process. The obstacles to overcome include the membrane fooling/clogging due to interactions with VOCs in NF/RO, fouling caused by high Fe concentration in MF/UF, and scaling in RO [4,13,63,64] as well as RO’s limitation to ionic strengths lower than that of sea water (approx. 40,000 ppm) [65].
- 4.
-
Electrocoagulation (EC) promotes the precipitation of metals in the form of hydroxides by the addition of direct current through a metal electrode. This has been shown to be efficient and economically feasible for wastewater [66]. Previous studies have demonstrated high removals of turbidity, COD, oils and greases by EC. For example, Kausley et al. reported efficacy in the removal of total organic carbon (TOC) and scaling-causing ions, particularly Ca2+, Mg2+, CO32− and HCO3-, from synthetic PW and PW [66,67]. The precipitation of metal cations in the form of hydroxides could be further exploited to make the treatment of FP more economically viable to the industrial sector through the generation and commercialization of Cu2+, Mn2+, Zn2+, Al3+, Fe3+, Ni2+, Mg2+, Ca2+, Na+ and several other metal hydroxides. Moreover, HCl could be produced by hydrolysis of Cl2 gas generated during the process [67,68,69,70]. (Greater details will be discussed in subsequent sections of this review).
- 5.
-
Distillation is a thermal process in which solid particles are separated from liquid matrix by boiling point differences. One of the promising variations for brine desalination is multistage flash distillation (MSF). In MSF, the saline solution is converted into a vapor state and then goes through successive units in which the solution evaporates and condensates. In each unit, a fraction of the original feed remains as a highly concentrated brine (see Figure 1) [62]. The technique produces high-quality fresh water [71] and is efficient in the treatment of brackish/sea water. Nevertheless, for future applications in PW treatment, it is suggested to pretreat the inlet water with chemical softeners, filtrations and/or ion exchange technologies to avoid scaling and fouling, as well as to upgrade the infrastructure material to stainless steel to prevent corrosion [62]. The latter increases capital costs. Additionally, the salts produced by this treatment modality can serve as a feedstock for electrocatalytic processes to produce acids (HCl) and caustic agents (NaOH).
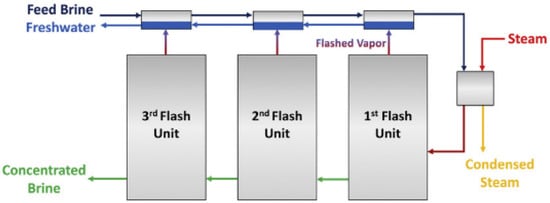 Figure 1. Schematics for multistage flash distillation (MSF)
Figure 1. Schematics for multistage flash distillation (MSF)
Many ongoing efforts for the treatment of FP incorporate separation and desalination [72]. Similarly, a common practice is the utilization of powdered activated carbon (PAC) for the depletion of dissolved organic carbon (DOC), turbidity and organic components. Other operations include softening hardness ions by the addition of caustic soda [54], demineralization through membrane distillation [73] and removal of organic components by coagulation followed by ultrafiltration [74]. Furthermore, biologically active membranes help remove organics and salinity [13]. The use of these techniques in tandem is generally required to remediate FP to a reusable and/or recyclable standard.
The commercial methods implemented in desalination of seawater, typically membrane-based and thermal-based [62], fail to meet the requirements for processing wastewater from UOG. However, the elevated values of TDS (>50,000) in FP can lead to difficult scenarios when treating the approximately 250 million barrels produced globally each day [75]. For example, the FP in the Permian Basin has TDS values three to five times higher when compared to those of seawater (see Table 1) [76]. Common challenges include corrosion, fouling and scaling of the membrane when precipitation conditions are met [77].
Forward osmosis allows the separation of water from dissolved solids by employing a semipermeable membrane and the difference in osmotic pressure as driving force. In contrast to RO, it is believed to be more appropriate for high-TDS matrices, such as FP [78]. Additionally, FO is a cost-competitive and reliable alternative for wastewater treatment [79] that exhibits great potential in removing heavy metal ions, including Cr2O72−, HAsO42−, Pb2+, Cd2+, Cu2+ and Hg2+ [80].
A previous study suggested that reusing PW in the energy sector is a better option than surface discharge due to safety concerns. Alternatively, its authors suggested thermal distillation (TD) as the appropriate treatment modality [42]. Regardless of being one of the most utilized operations for saline water recycling, TD’s energy consumption must be addressed when treating PW since scaling may lead to a to insulation of heat exchangers and, consequently, inefficient heat transfer. Again, the elevated price of anticorrosion materials to build this facility should be considered, since high costs affect the feasibility at an industrial scale. Similarly, osmotic properties constrain the application of membrane technologies in highly saline brines [62].
Recent advances in membrane technology, as well as integration of existing procedures, show promising results in processing high-TDS watersIn 2018, Sardari et al. demonstrated that electrocoagulation (EC) pre-treatment followed by direct contact membrane (DCMD) was effective in recovering up to 57% from a sample with a TDS of 135 g/L. However, they suggested a reduction in the sedimentation time for practical applications [81]. Furthermore, pretreatment with antiscalants such as 1-hydroxyethylidene-1,1-diphosphonic acid (HEDP) increased the performance of carbon-nanotube-immobilized membranes in membrane distillation (MD) [82]. Additionally, Ahmad et al. (2020) proposed a hybrid technology that incorporates assisted reverse osmosis (ARO), microfiltration and reverse osmosis—introduced as MF-ARO-RO—for which individual operations enhanced the ability to withstand different salinity effects and profiles. Although the addition of ARO to the MF-RO system represented an increase in the total cost, it was presented as the cheapest alternative for high-salinity FP [83].
Recent studies developed a combined membrane system consisting of an electrodialysis chamber followed by nanofiltration and membrane distillation (ED-NF-MD), represented in Figure 2. The system facilitated zero liquid discharge and allowed a water recovery of up to 99.8% with no need for chemical antiscalants [84]. Regardless of being a laboratory-scale experiment, the novel method has underlying potential in high-TDS waters treatment at industrial scale.
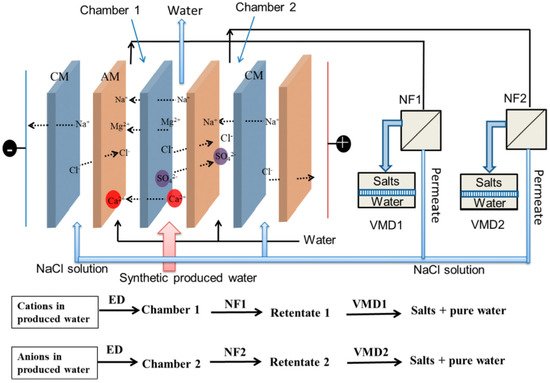
Figure 2. Diagram of the integrated ED-NF-MD system. CM: cation exchange membrane; AM: anion exchange membrane; ED: electrodialysis; NF: nanofiltration; VMD: vacuum membrane distillation.
To summarize, the utilization of different adsorbents and novel materials to prevent scaling and corrosion, as well as the tandem use of existing commercially available technologies, can facilitate the effective treatment of FP. Enhancement in the performance of the more sophisticated methods can be achieved by pretreatment with the well-known membrane filtrations.
2. Costs Associated with Produced Water Treatment
As previously mentioned, the efficacy of FP treatment is inherently important when determining the terminal destination for the treated water. However, the more influential aspect of assessing the feasibility and sustainability of FP reuse and/or recycling is operational cost. The cost of deep-well injections ranges from approximately USD $0.25/bbl in private wells to approx. USD $0.50 to 2.50/bbl in commercial wells [85]. Adding the price of transportation to disposal sites (approx. USD $0.03/bbl/mile) may increase the cost significantly depending on the location of the storage [86]. In fact, transportation costs can range from USD $2.00–20.00/bbl [87]. Moreover, these values are expected to become higher due to distances of disposal sites possibly increasing. Additionally, permitting SWDs is becoming more contentious because of earthquake issues could increase costs. On the other hand, FP can also be transported via pipeline at an approximate cost of USD $0.25/bbl (personal correspondence with water treatment provider), yet this requires considerable infrastructure that is generally not established in most shale energy basins. Typical treatment costs range from USD $3.00 to $30.00/bbl, including storage and transport [88]. Recently, MD modalities were studied for reuse waste waters of HF operations, resulting in costs ranging from USD $0.11 to $0.90/bbl of treated fluid [89]. Operational costs of RO and FO typically stand at USD ~$1.00/bbl. Providing an initial cost for the acquisition of these membranes is challenging due to their performance dependency on influent TDS levels and throughput requirements. In Table 2, the annual cost for FP disposal in Permian and Bakken is compared to treatment costs, assuming treatment take place in situ based on mobile treatment modalities.
Table 2. Annual cost for disposal and treatment of FP in Permian Basin and Bakken Shale, assuming both are performed on-site. * Based on USD $0.03/bbl/mile trucking cost and an average distance of 20 miles from the source to the nearest disposal site.
| Unit | Bakken Region | Permian Region | Reference | |
|---|---|---|---|---|
| Saltwater Disposal (SWD) cost | ||||
| Disposal volume | bbl/year | 3.43 × 108 | 1.6 × 109 | [41,42] |
| Transportation Cost * | USD/bbl | $0.60 | $0.60 | [86] |
| Well Injection Cost | USD/bbl | $0.5 | $0.5 | [43] |
| Well disposal Cost | USD/year | $171,730,000 | $831,605,000 | |
| Water management Cost—Scenario 1 | USD/year | $377.30 M | $1.76 B | |
| Treatment and reuse | ||||
| Chemical oxidation | USD/bbl | $0.20 | $0.20 | (Correspondence w/water treatment company) |
| Chemical precipitation & nanofiltration | USD/bbl | $0.24 | $0.24 | (Correspondence w/water treatment company) |
| Water management Cost—Scenario 2 | USD/year | $150.92 M | $704.00 M | |
This entry is adapted from the peer-reviewed paper 10.3390/en15134619
This entry is offline, you can click here to edit this entry!
