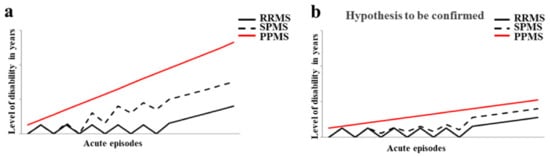
Multiple sclerosis (MS) is a neurological and inflammatory autoimmune disease of the central nervous system (CNS), in which selective activation of T and B lymphocytes prompts a reaction against myelin; these cells come from the peripheral circulation, penetrate the CNS and induce an inflammatory cascade resulting in demyelination and axonal loss. MS is characterized by the interruption of immunological self-tolerance to CNS myelin components, resulting in the rupture of myelin sheaths, clear inflammatory infiltrates, the proliferation of astrocytes, gliosis, the activation of microglia, axonal degeneration, oxidative stress, and mitochondrial damage [
1]. This neurologic disease predominantly affects young adults, with an incidence of 2.3 million people around the world, and mostly women at a ratio of 2:1 [
2,
3]. It is responsible for an array of symptoms involving the visual, sensory, motor, and autonomic systems. Optic neuritis (inflammation of the optic nerve) and Lhermitte’s phenomenon are initial and early symptoms characteristic of MS [
4,
5]. The age of MS onset is around 30 years old, but cases of pediatric or late MS can be found [
6,
7]. The most common phenotypes of MS are remittent relapsing MS (RRMS), which presents with an acute inflammatory episode and remittance with total or almost total recovery from each seizure. At the base of the inflammation, there is a massive involvement and increase in the lymphocyte population, activation of microglia, oxidative damage, mitochondrial injury, and energy failure [
8,
9,
10,
11]. About 15 years after diagnosis, up to 80% of people develop secondary progressive MS (SPMS), with a gradual increase in disability, marked neurodegenerative pathogenesis, and a reduced inflammatory state [
12]. Finally, there is primary progressive MS (PPMS), in which progressive disability occurs from the beginning, with massive involvement of the spinal cord, which is manifested by a progressive spastic paraparesis. The trend of disability in the different forms of MS is represented in
Figure 1a. The diagnosis of MS requires evidence of plaques in the areas of damage, within the white matter and the concomitant exclusion of other inflammatory, structural, or hereditary conditions that could give a similar clinical picture. In addition, it is characterized by increased latency of visually evoked potentials; the analysis of cerebrospinal fluid, obtained by lumbar puncture, which highlights the presence of oligoclonal bands with B cells that produce immunoglobulins; and magnetic resonance imaging (MRI) showing signal change areas [
13]. Until about 10 years ago, MS was considered a two-stage disease that involved early inflammation, responsible for the onset of the disease, in the form of RRMS; and delayed neurodegeneration responsible for the progression of the disease (SPMS), which led to more serious disabilities. Until 2010, the time between the first and the second phase was used for the administration of disease-modifying treatments (DMT) such as interferon beta, glatiramer acetate, and fingolimod (the first oral DMT), with conflicting results that often completely filled the temporal space preceding the progression of the disease and culminating in an irreversible disability [
14]. Today, a series of other drugs are available, including three monoclonal antibodies, which are administered earlier in order to avoid wasting that precious time that, from the initial stage, leads to the axonal loss responsible for permanent disability [
15]. In
Figure 1b, it is reported how the disease could be if it was treated early with the currently validated drugs. The involvement of environmental factors in the onset of MS is increasingly recognized, and this correlation is able to explain the epidemiological increase in the disease. Among these, the gut microbiota seems to have a relevant importance [
16].
Although MS is recognized as an autoimmune pathology of the central nervous system, the specific causes are many and thus, to date, it has been considered a disorder resulting from environmental factors in genetically susceptible individuals [
17]. It is precisely this variability of the causes of MS that determines the partial effectiveness of the drugs in use, each with a distinct immunomodulatory mechanism. Among the environmental factors hypothetically involved in MS are some viral infections, ethnicity, exposure to tobacco smoke, organic solvents, toxins or heavy metals, sun exposure, poor levels of vitamin D, obesity in adolescence, latitude and diet [
18]. Nutrition seems to be related to MS, although the role of nutritional factors is still unclear and further clinical trials are needed [
19]. The scientific literature obtained prior to 2005 demonstrated that the only correlations between nutrition and MS concerned the intake of macronutrients [
4]. For example, for a person affected with MS, an adequate intake of carbohydrates is very important because it allows them to maintain good energy levels and also counteract fatigue, a general symptom of the disease. Carbohydrates provide the energy needed to keep the body active without affecting sugar reserves (in the form of glycogen stored in the liver) and fat stocks. However, it is essential to reduce the intake of refined simple sugars, which increases the caloric content of food. Precisely for this reason, the diet of the patient with MS should never miss out on complex carbohydrates that provide energy by replacing them with protein to build muscle mass [
20]. Protein intake is important for people with MS for three reasons: (a) remedy for significant weight loss; (b) compensation for the decrease in muscle mass; (c) prevention of the possible formation of ulcers from decubitus, in the case of physical immobility [
21]. An excessive intake of saturated fats of animal origin could worsen the course of MS, alter the stability of the myelin sheath favoring demyelination [
22,
23]. Saturated fatty acids (SFAs) of animal origin must be controlled to avoid the increase in inflammatory processes in MS, and a low-fat diet may have some beneficial effects in the disease [
24,
25,
26]. Intake of SFAs increases LDL cholesterol, and this phenomenon is associated with a worsening of MS due to a direct impact on the immune system, activation of proinflammatory toll-like receptors, and increase in the proinflammatory transcription factor NF-kB [
27]. In a study using an animal model of MS, mice were fed a high-fat diet and increased T cells, inflammatory cytokine expression (IL-1β, IL-6, and IFNγ), and infiltrating macrophages were detected [
28]. The length of the fatty acid chain seems to be an extremely important factor in determining the progress of MS. In fact, while long-chain fatty acids promote the differentiation of proinflammatory T cells (TH1 and TH17) through the members of the MAP kinase family (an important action is carried out by P38 and JNK1), short-chain fatty acids (SCFAs) promote the differentiation of regulatory T cells through the production of anti-inflammatory cytokines [
29]. Recently, a study was published in which the correlation between fat intake and MS was highlighted: 219 young patients with a high intake of saturated fats had a threefold higher risk of developing new lesions compared to MS patients who did not consume as much fat [
30]. To date, it is known that minerals, antioxidants, trace elements, and vitamins can also be related to MS [
31]. For example, dietary antioxidants have important biological consequences in MS, since oxidative stress is one of the most important components of the inflammatory process, leading to the degradation of myelin and axonal damage [
32]. A recent study demonstrated that the consumption of foods with anti-inflammatory properties reduced the biological synthesis of proinflammatory molecules and improved the effectiveness of drugs with immunomodulatory activity [
33]. Polyunsaturated fatty acids (PUFAs) are characterized by multiple double bonds within the fatty acid chain and are found in foods such as fish, flax seed, and walnuts. PUFAs reduce inflammation through conversion into the anti-inflammatory prostaglandins E1 and E2, with effects on cytokine production, leukocyte migration, and other immune system components [
34]. In vivo studies have demonstrated that the administration of PUFAs was able to reduce the production of inflammatory cytokines, prevent demyelination, and promote remyelination [
35,
36]. Scientific studies have demonstrated, in vivo, that even a diet rich in salt determines adverse effects in MS: for example, a high amount of salt promotes the differentiation of proinflammatory TH17 cells, which develop a more pathogenic phenotype and a worsening of the course of the disease. In addition, Farez et al. found, in a study of 70 patients with RRMS, that those with a medium–high salt intake had clinical recurrence rates 3.95 times higher than those with low sodium intake [
37]. However, it is worth pointing out that these findings have been contradictory and that, currently, there are no further published clinical studies on the correlation of sodium intake and MS. On the contrary, increased fruit and vegetable intake has been associated with reduced levels of disease activity and disability [
38]. More specific studies have demonstrated that a diet rich in animal fat, milk, dairy products, meat, hydrogenated fats, and sugars and low in fruit, vegetables, and whole grains was related to a higher prevalence of MS and a higher level of disability [
39,
40]. In particular, an interesting study was conducted on 20 MS patients divided into two groups: 10 patients who consumed a diet characterized by high vegetable content and reduced protein (HV/LP), compared to 10 patients who ate a typical Western diet (WD) for a period of 12 months. The results obtained demonstrated that the HV/LP group had a reduction in proinflammatory T cells, a reduction in proinflammatory interleukins, and an increase in anti-inflammatory T lymphocytes [
41]. The pathophysiology of MS indicates that there are three factors on which to act in order to influence the course of the disease: (1) modulate inflammation; (2) protect against neurodegeneration; and (3) promote repair of the nervous system. It has been demonstrated that diet exerts a systemic influence on all three of these pathways, resulting in more or less beneficial effects [
42,
43]. A Mediterranean-style diet with a low content of saturated fat and processed foods, many polyunsaturated and monounsaturated fats, especially fish and olive oil, and plentiful fruits and vegetables has been associated in MS with reduced disability [
44], neurodegeneration [
45], and cognitive decay [
46]. In recent years, a close correlation between MS and vitamin levels has been highlighted. For example, the association between MS and vitamin D deficiency suggests that this vitamin may play a role in the immune response [
47]. Vitamin D, whose known forms include D2 or ergocalciferol and D3 or cholecalciferol, is taken either through exposure of the skin to sunlight or through the diet. The largest study related to this topic was carried out on over 7 million American soldiers and demonstrated an inverse correlation between serum levels of vitamin D and the risk of developing MS [
48]. Moreover, vitamin D seems to be not only a risk factor for the onset of MS, but also able to modulate the activity of the disease and its progression. In fact, low serum levels of vitamin D have been associated with an increase in disability, increased rate of recurrence, and an increase in the load of lesions, as evidenced by MRI [
49]. The pathophysiological mechanism of vitamin D responsible for the onset or progression of MS seems to be its role in the activation and proliferation of lymphocytes, the differentiation of T-helper cells, and its regulatory effects on the immune response [
50]. Vitamin D supplementation led to a reduction in CD4+ T cells producing IL-17, and the inhibition of the proliferation of B cells by induction of the apoptotic process [
51]. Several studies demonstrated the influence of other vitamins on MS: plasma concentrations of vitamin B12 and folate were decreased in patients with MS, due to their role in the formation of the myelin sheath [
52]. Finally, Bitarafan et al. determined that treatment with vitamin A improved cognitive ability and reduced disability in MS [
53]. The impact of various dietary factors in MS is very interesting and, for this reason, further preclinical models, epidemiological research, and prospective and clinical studies would be desirable. Therapy for MS cannot be replaced by a particular diet, but a healthy nutritional intervention can improve patients’ physical and inflammatory state.
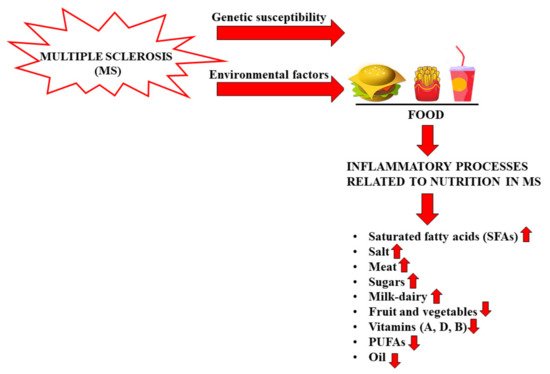
Figure 2 reports the effects of foods on inflammatory processes in MS.