Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Gelidium corneum (Giant Gelidium or Atlantic agar) is a well-known red seaweed harvested for its high-quality agar content. Agar is a mixture of the polysaccharides used in the food industry as a gelling, thickener, clarifying, and stabilizer agent. The best agar quality is also used in the laboratory as bacteriological agar.
- harvested biomass
- waste biomass
- Gelidium corneum applications
- Gelidium life cycle
- Gelidium cultivation
1. Introduction
Seaweeds are marine macroscopic photosynthetic organisms classified into three taxa, according to their main accessory pigment: red seaweeds (Rhodophyta) present phycoerythrin, green seaweeds (Chlorophyta) contain chlorophyll b, and brown seaweeds (Phaeophyceae) exhibit fucoxanthin [1]. These organisms occur mainly in coastal areas, whether rocky or sandy shores, salt marshes, swamps, lagoons, estuaries, or coral reefs, but also in deep-water and open water floating communities, distributed in tropical, temperate, and polar regions. Zonation and community distribution depend on different physical and chemical parameters such as temperature, substrate, salinity, pH, nutrients availability, hydrodynamics, light, tides, wind, and pollution level. Biological factors such as epiphytism, herbivory, and disease also shape the distribution of seaweeds [2].
Seaweeds are known for their healthy primary and secondary metabolite content, such as a high protein content with a balanced essential amino acid content, low lipid content, and high fibre and mineral content. They also contain hydrocolloids, pigments, fatty acids, polyphenols, vitamins, minerals, and terpenoids, and other biomolecules [3][4][5][6][7]. These bioactive compounds show a beneficial effect on human health and well-being, such as antioxidant, antimicrobial, anti-cancer, anti-inflammatory, and antithrombotic effects, and immune-system improvement, among others [8][9][10][11][12][13]. However, besides human welfare, there are many new biotechnological applications, including in the cosmetics industry, as a moisturizer, anti-ageing, and UV protector [14][15][16][17], in agriculture, as a biostimulant or biofertilizer [18][19][20][21], and in bioremediation and biosorption, due to the seaweeds’ ability to remove contaminants from the surrounding water [22][23][24]. Seaweed extracts are also gaining research interest as a bioplastic product due to the nature of their polymers, producing resistant durable biofilms [25][26]. Another current approach is the use of seaweed waste biomass to produce biofuels (biogas, methane, or bioethanol) since this process does not increase the atmospheric net CO2 [27][28]. First-generation biofuels are derived from vegetable oil, starch, or sucrose, usually derived from crops. Second-generation biofuels are derived from lignocellulosic waste biomass. Third-generation biofuels are produced from algae. The second- and third-generation biofuels are much more land-use and environmentally efficient than first-generation biofuels [29][30]. These applications depend on the quantity and nature of the biomolecules present in algal biomass. Thus, one of the key challenges is to be able to efficiently extract these bioactive compounds. Making use of all the constituents of the seaweeds, while keeping their characteristics and bioactivities intact, requires sequential processing of all the material, and often the purification of the algal by-products [31]. The concept of a biorefinery has been proposed allowing the extraction and, therefore, use of all useful components of seaweed in a cascade process, cost-effectively, adopting a zero-waste approach while reducing the impact on climate change [32]. Furthermore, in a circular economy framework, any economic activity must have a positive impact on the environment, and the market encourages the recycling of products rather than extracting new resources. In this production and consumption model, all forms of waste are returned to the economy and/or used more efficiently, allowing the life cycle of products to be significantly extended [33]. Therefore, in a circular economy context, seaweed biomass waste should be processed into new products, and many researchers are addressing this opportunity.
For many decades, one of the most industrially sought-after seaweeds was Gelidium. Gelidium sp. is a canopy-forming red seaweed (Rhodophyta) known for its high quality and content in agar [34][35][36][37][38]. Agar is a phycocolloid existing in the seaweed cell wall, consisting of a heterogeneous mixture of two polysaccharides, agarose, and agaropectin, the first with gelling properties and the second with thickening properties [39][40]. It is a semi-transparent, shiny, tasteless, odourless, and very hydrophilic colloid. Due to the formation of coiled helices, it forms very strong gels retaining water molecules when the agar solution is heated [41]. Gelidium sp. is the primary source of high-quality agar (high gelling strength and low sulphate content) and bacteriological grade agar, which is obtained only from this genus [2]. Although agar can be extracted from different species, such as Pterocladia, Pterocliadiella, Ahnfeltia, Acanthopeltis, and Gelidiella, the world agar market depends almost on wild-harvested Gelidium sp. and on cultivated Gracilaria sp., which produces a lower quality and lower price agar [42][43]. Currently, the most harvested agarophyte is the Gelidium corneum, being harvested in France, Italy, Portugal, Spain, and Morocco [44]. This wild harvest along with other human impacts, such as climate change, raises concern about the sustainability of the resource [45] and a global Gelidium landing shortage was recently diagnosed in 2018 [46]. Besides, the cultivation of Gelidium, although viable, did not reach enough yields to be economically profitable [47]. Hence, the management of the resource should be approached with attention.
Despite the importance of the agar market and the conservation concern, Gelidium sp. has been studied in the last two decades to evaluate other biomass properties such as antioxidant [48][49][50], antimicrobial [6][14][51][52], anti-inflammatory [53], antiproliferative or cytotoxic [54][55], biosorption of contaminants [56][57][58][59][60][61][62][63], and phyto-stimulant [64]. Besides, the use of agarophytic biomass has been proposed by several researchers for biorefinery, including in the energy sector [65][66][67]. These properties disclose the potential use of G. corneum in several applications for which different types of biomasses may be used: harvested, stranded, and waste. The use of harvested biomass for other purposes than as an agarophyte competes with the agar industry and raises serious management and conservation concerns. Yet, the stranded biomass and waste may also be suitable to incorporate into new bio-based materials. The agar industry, e.g., produces annually many tonnes of residual Gelidium biomass, which are treated as waste [68] or are used in the fertilizer industry with a very low commercial value [6][69]. However, this waste biomass can have other uses, and, thus, a better valorisation, adding value to this biomass already used industrially, and diversifying its use. This may create new business opportunities for coastal populations, who are economically dependent on this valuable natural resource.
2. Gelidium corneum Biology, Distribution, and Ecology
Gelidium corneum common names: Atlantic agar, Giant Gelidium (English), Gelidium imperial (French), Ágar, Limo-encarnado, Cabelo-de-cão (Portuguese), Ocle, Caloca (Spanish) [70][71].
The Genus Gelidium includes, currently, 144 taxonomically accepted marine species [72] distributed worldwide. Among these species, Gelidium corneum (Hudson) J.V. Lamouroux (formerly Gelidium sesquipedale) is one of the best-known species. It is a cartilaginous dark-red seaweed (division Rhodophyta), with flattened branches with spoon-shaped branchlets, and creeping stolons at the base, up to 20 (30) centimetres tall, forming large tufts. Erect thalli grow from a system of creeping axes attached to rocky substrates through rhizoids (Figure 1) [69][70][73][74][75].

Figure 1. Macroscopic image of Gelidium corneum collected in Centre Portugal.
Gelidium is a clonal-modular seaweed [2]; consequently, it spreads laterally and vegetatively over the soil surface via creeping axes. It also produces erect thalli (fronds). Storms and grazing remove the fronds but not the creeping axes, which remain attached to the substrate. Regeneration and growth of erect fronds from the creeping axes are common and fast [76]. An interesting feature of Gelidium populations is the ability to grow from vegetative reproduction. These erect fronds can have an autonomous life when fragmented and can reattach to the substrate, so vegetative propagation, through fragmentation, is a frequent method of colonisation [76]. The species has a wide distribution, occurring in Atlantic Europe [6][73][77][78][79][80][81][82], Mediterranean Sea [83], Atlantic islands [84][85][86], Atlantic Africa [87][88][89][90], and Atlantic America [91]. Guiry also mentions populations in the Indic and Pacific oceans, namely in India, Indonesia, Korea, Vietnam, and Australia, but no published information on these locations could be found [72] (Figure 2).
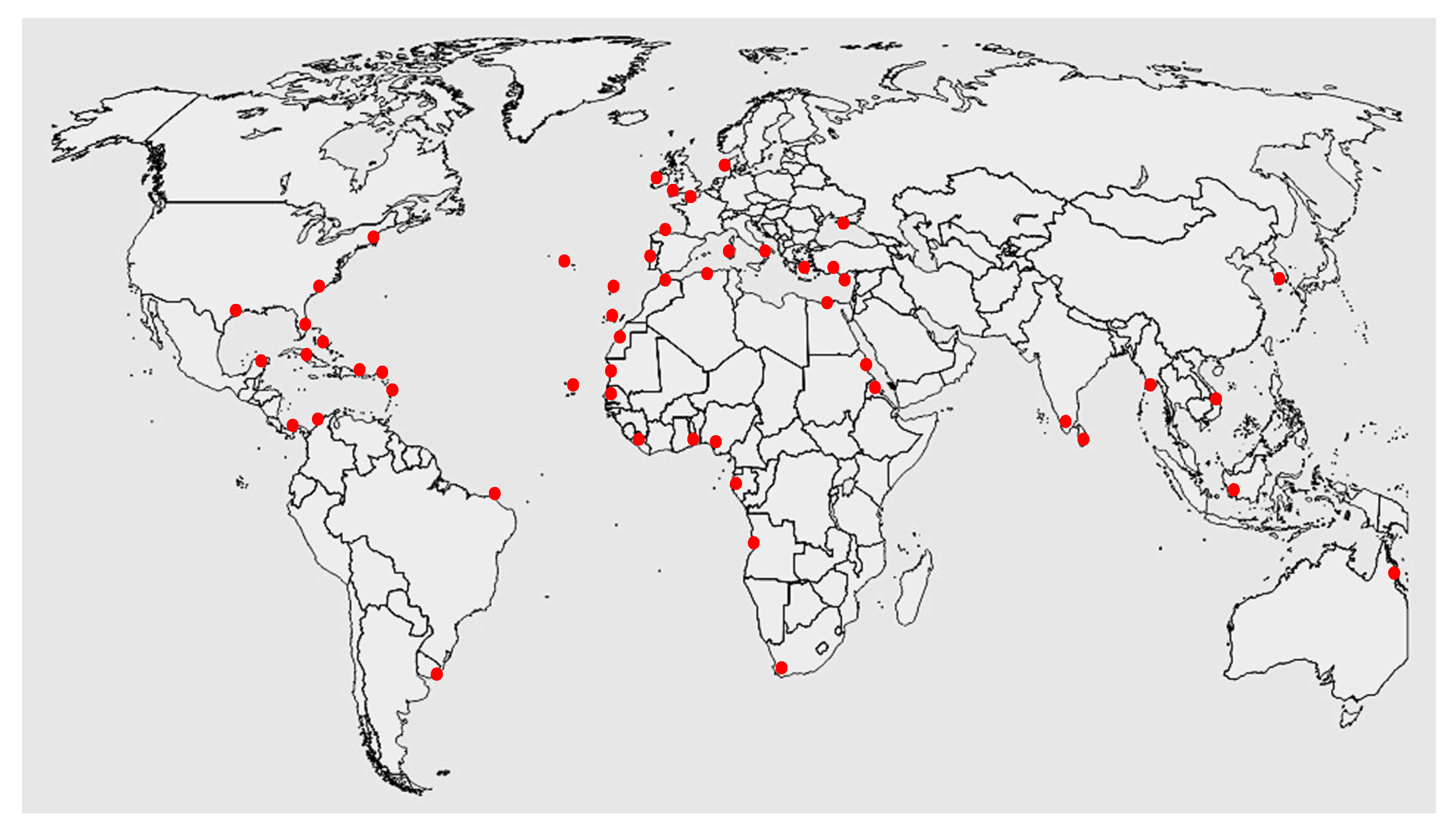
Figure 2. World distribution of Gelidium corneum (data from Guiry [72]).
G. corneum grows in temperate to tropical areas, with seasonal temperatures ranging between 10 and 25 °C, in partly shaded habitats, with strong tides and sea currents. In Europe, G. corneum forms widespread beds, usually subtidal zones up to about 25 m in depth. As to the substrate, the species prefers growing on slightly sloping regular bedrock, with little to moderate sand sedimentation [77][92]. In these temperate waters, Gelidium species reach high abundances and frequencies. Regardless of the species or the latitude, these are slow-growing organisms, up to 100 mm y−1 [76][93][94]. G. corneum is a canopy-forming seaweed, which is to say, it is a habitat-forming seaweed, creating a stable and complex community providing food, shelter, nursery, and habitat for many other species, such as invertebrates, fish, and other smaller algae [95][96]. G. corneum is sensitive to environmental parameters such as temperature, light, nutrients, and water movement [97]. Santelices [76] also states several biological factors affecting productivity, comprising morphology, age of the fronds, thallus part, reproductive state, seasonality, crop density, life history phase, and geographic and ecological origin of the species. Additional events of importance affecting Gelidium populations include extreme low tides, storms, and grazing [36][76].
In many areas of the globe, the cumulative impacts of human pressure, such as habitat destruction, pollution, over-harvesting, invasive species, and ocean warming, decrease the resilience of the seaweeds and promote the loss of the seaweed’s biomass. G. corneum is no exception, showing a decline in the past decades. In response to these disruptions, shifts in the distribution patterns of canopy species occur. Notably, these shifts are observed with a decline in canopy-forming species, the increase in morphologically simpler warm-water species and coralline algae, and the progressive introduction and expansion of non-indigenous species [45][78][79][98][99][100][101][102][103][104][105].
3. Gelidium corneum Life Cycle
The genus has a complex life cycle, representative of the most evolved red seaweeds (class Florideophyceae), with a triphasic isomorphic life cycle [74]. This life cycle is portrayed by a haploid independent gametophyte, producing gametes through mitosis (either male spermatangia or female carpogonia). The male gametes (spermatia) are released and pass to the trichogyne of the female gamete (carpogonium) where fertilization occurs (Figure 3). The mitotic division of the zygote produces gonimoblast filaments, the first diploid generation (carposporophyte phase), which grow within the female gametophytes. These carposporophytes produce carposporangia inside which diploid carpospores are formed by mitosis. Carpospores are released and each matures into an independent diploid tetrasporophyte, the second diploid generation. The tetrasporangial mother cell divides by meiosis to produce four haploid tetraspores, each becoming a new gametophyte [106]. Although isomorphic, the tetrasporangial are more common than the gametangial thalli. This finding suggests that tetrasporophytes are more robust and competitive and the gametophytes are more sensitive to environmental conditions and less viable [76].
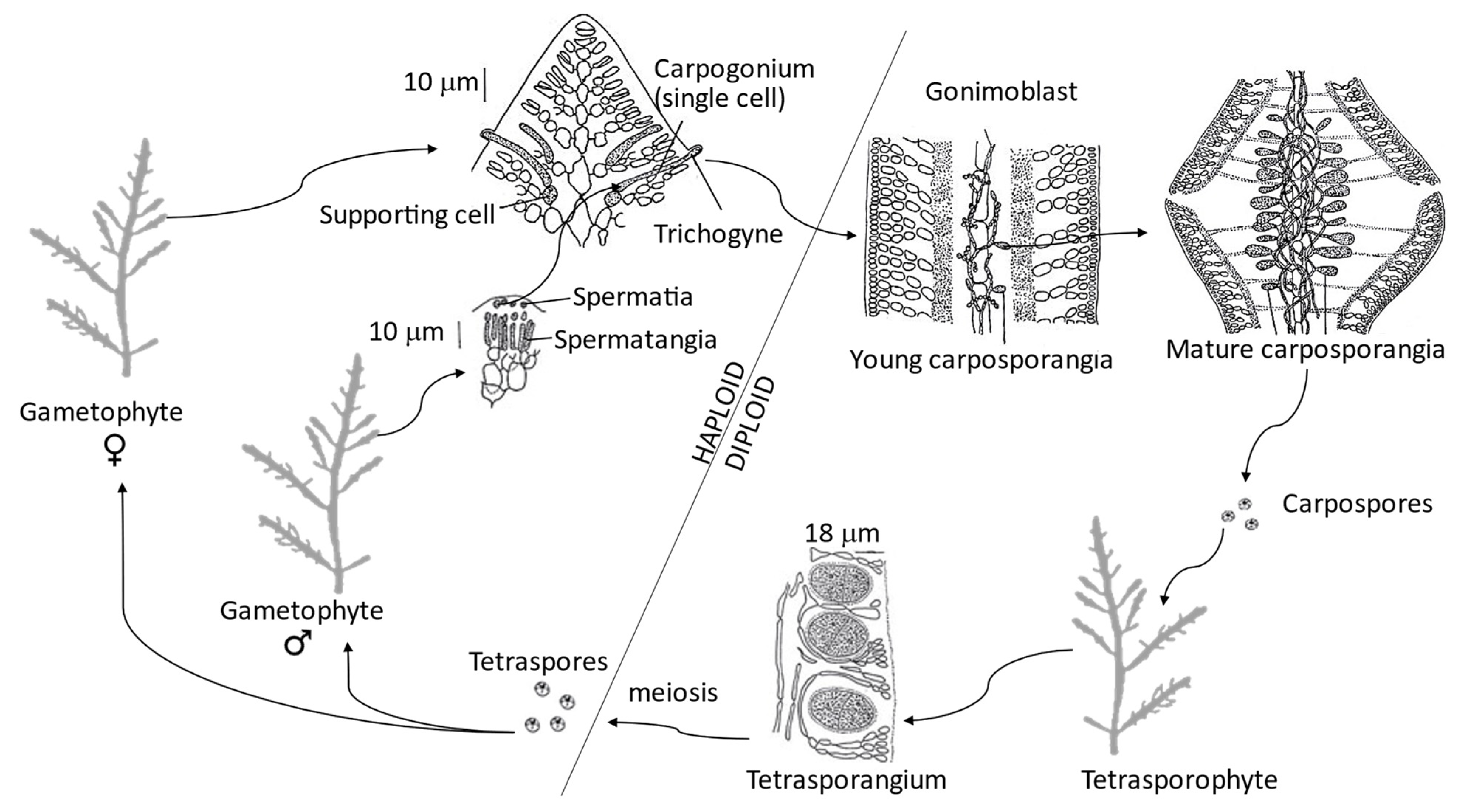
Figure 3. Triphasic isomorphic haplodiploid life cycle of Gelidium corneum, adapted from Lee [106].
The species is reported to have a certain inability to attach itself to a substrate through spore colonisation. So, sexual reproduction is often replaced by vegetative reproduction, in which the creeping thalli produce a disk of rhizoids that can attach and penetrate suitable substrates [107].
4. Gelidium Harvesting
Different species of Gelidium are harvested in the world to produce agar. G. corneum is harvested in Portugal, Spain, France, and Morocco, G. amansii and G. latifolium in Japan, Taiwan, Korea, and Indonesia, and G. robustum in Mexico. Smaller quantities of G. lingulatum, G. chilense, and G. rex are harvested in Chile, whereas G. pristoides, G. abbottiorum G. pteridifolium, G. capense are harvested in China, Namibia, and South Africa [46][92][108][109]. The biomass may be gathered from beach-cast seaweeds, using nets or rakes, a method common in countries such as South Africa, Australia, and New Zealand [110]. The attached thalli may also be cut by hand. More recently, seaweeds are harvested mechanically with the biomass being plucked off by divers, stowing the seaweeds in bags or baskets which are then lifted onto a boat [95][111]. When the creeping axes are removed, regeneration of the vertical thalli may take several years to regenerate, allowing the area to be invaded by other algae [94]. Harvesting, therefore, has a direct impact on the biomass and structure of seaweed beds and marine biodiversity. Harvesting canopy-forming seaweeds affects the morphology, canopy structure, thalli growth and regeneration, standing stock, and species composition of the foundation species. In turn, these changes affect the ecological roles of the canopy-forming seaweeds in marine ecosystems [110].
No doubt wild seaweed harvesting is facing the challenge of balancing the socio-economic and environmental sustainability of the activity. The impact on the wild stocks depends on the methods used, the mechanical clear-cutting being more severe than the hand-harvesting. For G. corneum, the creeping taxa must be left unharmed, allowing Gelidium populations to recover rapidly [94]. Over the past decades, management of natural resources has tackled the need to protect species and ecosystems, enabling habitat protection to sustain species diversity and abundance, whilst simultaneously granting sustainable exploitation of the marine resources. There is a need to evaluate permanent stocks of Gelidium where they are still harvested, to define harvest effort, to study the possibility of restocking, to establish and respect the harvest season, and to apply local resource management regulations [110].
5. Gelidium Cultivation
The demand for algal biomass for industrial purposes has far outstripped the capacity that traditional harvesting of wild stocks can provide. Trying to meet the demand, different attempts to cultivate Gelidium species have been performed for the past decades with some biological success but without economical relevance due to the slow growth of the genus [93]. These attempts were carried out in the laboratory, inland (tanks or ponds), or in the sea (net bag method, rafts, and net pouch), forming spores, fragments, and grown from reattachment thalli [74][107][112][113][114][115][116][117][118][119]. Different cultivation conditions were tested, assessing the influence of temperature, season, irradiance, nutrients, and water movement productivity of the biomass [94][117][120][121][122][123]. Although the experiments succeeded to a greater or lesser extent, they resulted in low yields. Yields vary among species, but cultivation points to a maximum yield of 25 kg FW m−2, which cannot compete economically with wild harvesting [47]. The only species that has been industrially produced is G. amansii in ponds, recorded for North Korea, but with very little data available [46][124].
Despite these setbacks, G. corneum is an important biological resource with multiple applications, so the feedstock cannot depend exclusively on wild resources. Further scientific research is, therefore, required to develop more efficient cultivation techniques that will produce abundant quality biomass at a competitive price compared to wild biomass. Therefore, to introduce economically profitable cultivation of Gelidium, it will be required to work on genetic improvement of the genus, through the selection of the more productive strains or genetic engineering [47]. Genetic engineering, together with further studies on cultivation and reproduction techniques, will hopefully allow full domestication of Gelidium, as has already been achieved for other seaweed species in the past years.
This entry is adapted from the peer-reviewed paper 10.3390/earth3030045
References
- Baweja, P.; Kumar, S.; Sahoo, D.; Lewiston, M. Biology of Seaweeds. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press: London, UK; Elsevier: London, UK, 2016; ISBN 9780128027721.
- Hurd, C.L.; Harrison, P.J.; Bischof, K.; Lobban, C.S. Seaweed Ecology and Physiology, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; ISBN 978-0521145954.
- Pereira, L. A Review of the Nutrient Composition of Selected Edible Seaweeds. In Seaweed: Ecology, Nutrient Composition and Medicinal Uses; Pomin, V.H., Ed.; Nova Science Publishers, Inc.: Coimbra, Portugal, 2011; Volume 4, pp. 15–47. ISBN 978-1-61470-878-0.
- Macartain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional Value of Edible Seaweeds. Nutr. Rev. 2007, 65, 535–543.
- Patarra, R.F.; Paiva, L.; Neto, A.I.; Lima, E.; Baptista, J. Nutritional Value of Selected Macroalgae. J. Appl. Phycol. 2011, 23, 205–208.
- Cavaco, M.; Duarte, A.; Freitas, M.V.; Afonso, C.; Bernardino, S.; Pereira, L.; Martins, M.; Mouga, T. Seasonal Nutritional Profile of Gelidium Corneum (Rhodophyta, Gelidiaceae) from the Center of Portugal. Foods 2021, 10, 2394.
- Afonso, C.; Correia, A.P.; Freitas, M.V.; Baptista, T.; Neves, M.; Mouga, T. Seasonal Changes in the Nutritional Composition of Agarophyton Vermiculophyllum (Rhodophyta, Gracilariales) from the Center of Portugal. Foods 2021, 10, 1145.
- Burtin, P. Nutricional Value of Seaweeds. Electron. J. Environ. Agric. Food Chem. 2003, 2, 498–503.
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding. J. Appl. Phycol. 2017, 29, 949–982.
- de Jesus Raposo, M.; de Jesus Raposo, M.F.; de Morais, A.M.B.; de Morais, R.M.S.C. Marine Polysaccharides from Algae with Potential Biomedical Applications. Mar. Drugs 2015, 13, 2967–3028.
- Pádua, D.; Rocha, E.; Gargiulo, D.; Ramos, A.A. Bioactive Compounds from Brown Seaweeds: Phloroglucinol, Fucoxanthin and Fucoidan as Promising Therapeutic Agents against Breast Cancer. Phytochem. Lett. 2015, 14, 91–98.
- Wijesinghe, W.A.J.P.; Jeon, Y.J. Biological Activities and Potential Industrial Applications of Fucose Rich Sulfated Polysaccharides and Fucoidans Isolated from Brown Seaweeds: A Review. Carbohydr. Polym. 2012, 88, 13–20.
- Hafting, J.T.; Craigie, J.S.; Stengel, D.B.; Loureiro, R.R.; Buschmann, A.H.; Yarish, C.; Edwards, M.D.; Critchley, A.T. Prospects and Challenges for Industrial Production of Seaweed Bioactives. J. Phycol. 2015, 51, 821–837.
- Matias, M.; Pinteus, S.; Martins, A.; Silva, J.; Alves, C.; Mouga, T.; Gaspar, H.; Pedrosa, R. Gelidiales Are Not Just Agar—Revealing the Antimicrobial Potential of Gelidium Corneum for Skin Disorders. Antibiotics 2022, 11, 481.
- Freitas, M.V.; Pacheco, D.; Cotas, J.; Mouga, T.; Afonso, C.; Pereira, L. Red Seaweed Pigments from a Biotechnological Perspective. Phycology 2021, 2, 1–29.
- Pandey, A.; Pandey, S.; Pathak, J.; Ahmed, H.; Singh, V.; Singh, S.P.; Sinha, R.P. Mycosporine-Like Amino Acids (MAAs) Profile of Two Marine Red Macroalgae, Gelidium sp. and Ceramium sp. Int. J. Appl. Sci. Biotechnol. 2017, 5, 12–21.
- Pimentel, F.; Alves, R.; Rodrigues, F.; PP Oliveira, M. Macroalgae-Derived Ingredients for Cosmetic Industry—An Update. Cosmetics 2017, 5, 2.
- Hernández Carmona, G. Seaweed as Potential Plant Growth Stimulants for Agriculture in Mexico. Hidrobiológica 2018, 28, 129–140.
- Villares, R.; Fernández-Lema, E.; López-Mosquera, M.E. Evaluation of Beach Wrack for Use as an Organic Fertilizer: Temporal Survey in Different Areas. Thalassas 2016, 32, 19–36.
- Ali, M.K.M.; Critchley, A.T.; Hurtado, A.Q. The Impacts of AMPEP K+ (Ascophyllum Marine Plant Extract, Enhanced with Potassium) on the Growth Rate, Carrageenan Quality, and Percentage Incidence of the Damaging Epiphyte Neosiphonia Apiculata on Four Strains of the Commercially Importan. J. Appl. Phycol. 2020, 32, 1907–1916.
- Hurtado, A.Q.; Neish, I.C.; Majahar Ali, M.K.; Norrie, J.; Pereira, L.; Michalak, I.; Shukla, P.S.; Critchley, A.T. Extracts of Seaweeds Used as Biostimulants on Land and Sea Crops—An Efficacious, Phyconomic, Circular Blue Economy: With Special Reference to Ascophyllum (Brown) and Kappaphycus (Red) Seaweeds. In Biostimulants for Crops from Seed Germination to Plant Development; Shubhpriya, G., van Staden, J., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2021; Volume 1, pp. 263–288. ISBN 9781119130536.
- Roberts, D.A.; Paul, N.A.; Dworjanyn, S.A.; Bird, M.I.; de Nys, R. Biochar from Commercially Cultivated Seaweed for Soil Amelioration. Sci. Rep. 2015, 5, 9665.
- Grebe, G.S.; Byron, C.J.; Gelais, A.S.; Kotowicz, D.M.; Olson, T.K. An Ecosystem Approach to Kelp Aquaculture in the Americas and Europe. Aquac. Rep. 2019, 15, 100215.
- Zeraatkar, A.K.; Ahmadzadeh, H.; Talebi, A.F.; Moheimani, N.R.; McHenry, M.P. Potential Use of Algae for Heavy Metal Bioremediation, a Critical Review. J. Environ. Manag. 2016, 181, 817–831.
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Camarena-Gómez, M.T.; Piiparinen, J.; Spilling, K.; Yang, B. European Union Legislation on Macroalgae Products. Aquac. Int. 2021, 29, 487–509.
- Diop, C.I.K.; Trigueros, E.; Sanz, M.T.; Beltran, S.; García-Tojal, J. Pressurized Hot Water-Assisted Recovery of Crude Residual Agar from a Never-Dried Algae Industry Waste Stream: A Box-Behnken Design Approach. Food Hydrocoll. 2022, 129, 107664.
- Fasahati, P.; Dickson, R.; Saffron, C.M.; Woo, H.C.; Liu, J.J. Seaweeds as a Sustainable Source of Bioenergy: Techno-Economic and Life Cycle Analyses of Its Biochemical Conversion Pathways. Renew. Sustain. Energy Rev. 2022, 157, 112011.
- Thakur, N.; Salama, E.S.; Sharma, M.; Sharma, P.; Sharma, D.; Li, X. Efficient Utilization and Management of Seaweed Biomass for Biogas Production. Mater. Today Sustain. 2022, 18, 100120.
- Cherubini, F. The Biorefinery Concept: Using Biomass Instead of Oil for Producing Energy and Chemicals. Energy Convers. Manag. 2010, 51, 1412–1421.
- Offei, F.; Mensah, M.; Thygesen, A.; Kemausuor, F. Seaweed Bioethanol Production: A Process Selection Review on Hydrolysis and Fermentation. Fermentation 2018, 4, 99.
- Torres, M.D.; Kraan, S.; Domínguez, H. Seaweed Biorefinery. Rev. Environ. Sci. Bio/Technol. 2019, 18, 335–388.
- Schiener, P.; Atack, T.; Wareing, R.; Kelly, M.S.; Hughes, A.D. The By-Products from Marine Biofuels as a Feed Source for the Aquaculture Industry: A Novel Example of the Biorefinery Approach. Biomass Convers. Biorefinery 2016, 6, 281–287.
- Guillot, J.D. Circular Economy: Definition, Importance and Benefits. Available online: https://www.europarl.europa.eu/news/en/headlines/economy/20151201STO05603/circular-economy-definition-importance-and-benefits (accessed on 25 May 2022).
- Martínez-Sanz, M.; Gomez-Barrio, L.P.; Zhao, M.; Tiwari, B.; Knutsen, S.H.; Ballance, S.; Zobel, H.K.; Nilsson, A.E.; Krewer, C.; Östergren, K.; et al. Alternative Protocols for the Production of More Sustainable Agar-Based Extracts from Gelidium Sesquipedale. Algal Res. 2021, 55, 102254.
- Martínez-Sanz, M.; Gómez-Mascaraque, L.G.; Ballester, A.R.; Martínez-Abad, A.; Brodkorb, A.; López-Rubio, A. Production of Unpurified Agar-Based Extracts from Red Seaweed Gelidium Sesquipedale by Means of Simplified Extraction Protocols. Algal Res. 2019, 38, 101420.
- Santos, R.; Cristo, C.; Jesus, D. Stock Assessment of the Agarophyte Gelidium Sesquipedale Using Harvest Effort Statistics. In Proceedings of the 17th International Seaweeds Symposium; Chapman, A., Anderson, R., Vreeland, V., Davison, I., Eds.; Oxford University Press: Cape Town, South Africa, 2001; pp. 145–150.
- Nil, S.; Ali-Mehidi, S.; Zellal, A.; Abi-Ayad, S.M.E.A. Effects of Season on the Yield and Quality of Agar from Gelidium Sesquipedale (Rhodophyta) from Mostaganem, Algeria. Afr. J. Biotechnol. 2016, 15, 350–355.
- Bellatmania, Z.; Bentiss, F.; Jama, C.; Nadri, A.; Reani, A.; Sabour, B.; Belattmania, Z. Spectroscopic Characterization and Gel Properties of Agar from Two Gelidium Species from the Atlantic Coast of Morocco. Biointerface Res. Appl. Chem. 2021, 2021, 12642–12652.
- Cardoso, S.S.M.; Carvalho, L.L.G.L.; Silva, P.P.J.; Rodrigues, M.S.M.; Pereira, O.; Pereira, L. Bioproducts From Seaweeds: A Review With Special Focus On The Iberian Peninsula. Curr. Org. Chem. 2014, 18, 896–917.
- Williams, P. Food Polysaccharides and Their Applications, 2nd ed.; Stephen, A.M., Phillips, G.O., Williams, P.A., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2006; ISBN 9780824759223.
- Kim, S.-K. Handbook of Marine Macroalgae—Biotechnology and Applied Phycology, 1st ed.; Kim, S.-K., Ed.; John Wiley & Sons: West Sussex, UK, 2012; ISBN 9780470979181.
- Bixler, H.J.; Porse, H. A Decade of Change in the Seaweed Hydrocolloids Industry. J. Appl. Phycol. 2011, 23, 321–335.
- Gallardo, T. Marine Algae: General Aspects (Biology, Systematics, Field and Laboratory Techniques). In Marine Algae Biodiversity, Taxonomy, Environmental Assessment, and Biotechnology; Pereira, L., Neto, J.M., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Boca Raton, FL, USA, 2015; ISBN 9781466581814.
- FAO Fishery and Aquaculture Statistics. Global Capture Production 1950–2020 (FishStatJ). Available online: https://www.fao.org/fishery/en/statistics/software/fishstatj/en (accessed on 19 May 2022).
- Borja, Á.; Fontán, A.; Muxika, I. Interactions between Climatic Variables and Human Pressures upon a Macroalgae Population: Implications for Management. Ocean. Coast. Manag. 2013, 76, 85–95.
- Santos, R.; Melo, R.A. Global Shortage of Technical Agars: Back to Basics (Resource Management). J. Appl. Phycol. 2018, 30, 2463–2473.
- Friedlander, M. Advances in Cultivation of Gelidiales. J. Appl. Phycol. 2008, 20, 451–456.
- Trigueros, E.; Sanz, M.T.; Filipigh, A.; Beltrán, S.; Riaño, P. Enzymatic Hydrolysis of the Industrial Solid Residue of Red Seaweed after Agar Extraction: Extracts Characterization and Modelling. Food Bioprod. Processing 2021, 126, 356–366.
- Grina, F.; Ullah, Z.; Kaplaner, E.; Moujahid, A.; Eddoha, R.; Nasser, B.; Terzioğlu, P.; Yilmaz, M.A.; Ertaş, A.; Öztürk, M.; et al. In Vitro Enzyme Inhibitory Properties, Antioxidant Activities, and Phytochemical Fingerprints of Five Moroccan Seaweeds. South Afr. J. Bot. 2020, 128, 152–160.
- Trommer, H.; Neubert, R.H.H. The Examination of Polysaccharides as Potential Antioxidative Compounds for Topical Administration Using a Lipid Model System. Int. J. Pharm. 2005, 298, 153–163.
- Lakhdar, F.; Sidi, O.A.; Ben, M. Antimicrobial Effect of Two Marine Algae Gelidium Sesquipedale and Laminaria Ochroleuca Collected from the Coast of El Jadida-Morocco. J. Bio. Innov. 2016, 5, 16–23.
- Öztürk, B.Y.; Gürsu, B.; Dağ, İ. Antibiofilm and Antimicrobial Activities of Green Synthesized Silver Nanoparticles Using Marine Red Algae Gelidium Corneum. Process Biochem. 2020, 89, 208–219.
- Matos, J.; Gomes, A.; Cardoso, C.; Afonso, C.; Campos, A.M.; Gomes, R.; Falé, P.; Delgado, I.; Coelho, I.; Castanheira, I.; et al. Commercial Red Seaweed in Portugal (Gelidium Sesquipedale and Pterocladiella Capillacea, Florideophyceae): Going beyond a Single-Purpose Product Approach by Valorizing Bioactivity. Thalassas 2020, 36, 213–224.
- Metidji, H.; Dob, T.; Toumi, M.; Krimat, S.; Ksouri, A.; Nouasri, A. In Vitro Screening of Secondary Metabolites and Evaluationof Antioxidant, Antimicrobial and Cytotoxic Properties of Gelidium Sesquipedale Thuret et Bornet Red Seaweed from Algeria. J. Mater. Environ. Sci. 2015, 6, 3184–3196.
- Grozdanic, N.; Stanojkovic, T.P.P.; Kljajic, Z.; Etahiri, S.; Assobhei, O.; Konic-Ristic, A.; Srdic-Rajic, T.; Kardum, N.; Backovic, S.; Osmak, M.; et al. In Vitro Evaluation of Antioxidant and Antitumoral Activities of Marine Algae Gelidium Sesquipedale and Fucus Spiralis. Eur. J. Cancer 2012, 48, S26.
- Vilar, V.; Botelho, C.; Boaventura, R. Biosorption Performance of a Binary Metal Mixture by Algal Biomass: Column Experiments. In Combined and Hybrid Adsorbents; Loureiro, J.M., Kartel, M.T., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 281–286.
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Methylene Blue Adsorption by Algal Biomass Based Materials: Biosorbents Characterization and Process Behaviour. J. Hazard. Mater. 2007, 147, 120–132.
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Equilibrium and Kinetic Modelling of Cd(II) Biosorption by Algae Gelidium and Agar Extraction Algal Waste. Water Res. 2006, 40, 291–302.
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Copper Desorption from Gelidium Algal Biomass. Water Res. 2007, 41, 1569–1579.
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Metal Biosorption by Algae Gelidium Derived Materials from Binary Solutions in a Continuous Stirred Adsorber. Chem. Eng. J. 2008, 141, 42–50.
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Lead Uptake by Algae Gelidium and Composite Material Particles in a Packed Bed Column. Chem. Eng. J. 2008, 144, 420–430.
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Copper Removal by Algae Gelidium, Agar Extraction Algal Waste and Granulated Algal Waste: Kinetics and Equilibrium. Bioresour. Technol. 2008, 99, 750–762.
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Effect of Cu(II), Cd(II) and Zn(II) on Pb(II) Biosorption by Algae Gelidium-Derived Materials. J. Hazard. Mater. 2008, 154, 711–720.
- Lebbar, S. Valorisation Biologique de Co-Produits de l’extraction de l’agar Issu Du Gelidium Sesquipedale. Ph.D. Thesis, Cadre de École doctorale Chimie, Ecologie, Géosciences et AgroSciences Théodore Monod (Poitiers), Limoges, France, 2018.
- Francavilla, M.; Manara, P.; Kamaterou, P.; Monteleone, M.; Zabaniotou, A. Cascade Approach of Red Macroalgae Gracilaria Gracilis Sustainable Valorization by Extraction of Phycobiliproteins and Pyrolysis of Residue. Bioresour. Technol. 2015, 184, 305–313.
- Baghel, R.S.; Trivedi, N.; Gupta, V.; Neori, A.; Reddy, C.R.K.; Lali, A.; Jha, B. Biorefining of Marine Macroalgal Biomass for Production of Biofuel and Commodity Chemicals. Green Chem. 2015, 17, 2436–2443.
- Kumar, S.; Gupta, R.; Kumar, G.; Sahoo, D.; Kuhad, R.C. Bioethanol Production from Gracilaria Verrucosa, a Red Alga, in a Biorefinery Approach. Bioresour. Technol. 2013, 135, 150–156.
- Baghel, R.S.; Reddy, C.R.K.; Singh, R.P. Seaweed-Based Cellulose: Applications, and Future Perspectives. Carbohydr. Polym. 2021, 267, 118241.
- Cavaco, M.; Duarte, A.; Bernardino, S.; Afonso, C.; Mouga, T. Sustainable Use of Seaweeds from S. Martinho Do Porto, Portugal—Past, Present, and Future Perspective. In Proceedings of the 1st International Conference on Water Energy Food and Sustainability (ICoWEFS 2021); Galvão, J., Brito, P., Neves, F., Craveiro, F., Almeida, H., Vasco, J., Neves, L., Gomes, R., Mourato, S., Ribeiro, V., Eds.; Springer Nature: Leiria, Portugal, 2021; pp. 1–11.
- Pereira, L. Edible Seaweeds of the World; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781498730501.
- FAO Fisheries and Aquaculture—List of Species for Fishery Statistics Purposes—Gelidium Corneum. Available online: https://www.fao.org/fishery/en/species/18380 (accessed on 19 May 2022).
- Guiry, M.D.; AlgaeBase. World-Wide Electronic Publication, National University of Ireland. Available online: www.Algaebase.Org (accessed on 26 April 2022).
- Bunker, F.; Brodie, J.A.; Maggs, C.A.; Bunker, A.R. Seaweeds of Britain and Ireland, 2nd ed.; Wild Nature Press: Plymouth, MA, USA, 2017; ISBN 978-0-9955673-3-7.
- Santelices, B. Synopsis of Biological Data on the Seaweed Genera Gelidium and Pterocladia; FAO: Rome, Italy, 1988; ISBN 925102717X.
- Silva, J.; Santos, R. Comparative Ecophysiology of Gelidium Sesquipedale (Rhodophyta) Erect Fronds and Prostrate System. In Proceedings of the 17th International Seaweed Symposium; Chapman, A., Anderson, R., Vreeland, V., Davison, I., Eds.; Oxford University Press: Oxford, UK, 2001; pp. 1–8.
- Santelices, B. Production Ecology of Gelidium. Hydrobiologia 1991, 221, 31–44.
- Gorostiaga, J.M.; Santolaria, A.; Secilla, A.; Casares, C. Check-List of the Basque Coast Benthic Algae (North of Spain). An. Del Jardín Botánico De Madr. 2004, 61, 155–180.
- Díez, I.; Santolaria, A.; Gorostiaga, J.M. The Relationship of Environmental Factors to the Structure and Distribution of Subtidal Seaweed Vegetation of the Western Basque Coast (N Spain). Estuar. Coast. Shelf Sci. 2003, 56, 1041–1054.
- Díez, I.; Muguerza, N.; Santolaria, A.; Ganzedo, U.; Gorostiaga, J.M. Seaweed Assemblage Changes in the Eastern Cantabrian Sea and Their Potential Relationship to Climate Change. Estuar. Coast. Shelf Sci. 2012, 99, 108–120.
- Burel, T.; Le Duff, M.; Gall, E.A. Updated Check-List of the Seaweeds of the French Coasts, Channel and Atlantic Ocean. Les Cah. Nat. De L’observatoire Mar. 2019, 7, 1–38.
- Araújo, R.; Bárbara, I.; Tibaldo, M.; Berecibar, E.; Tapia, P.D.; Pereira, R.; Santos, R.; Pinto, I.S. Checklist of Benthic Marine Algae and Cyanobacteria of Northern Portugal. Bot. Mar. 2009, 52, 24–46.
- Dizerbo, A.H. La Répartition Géographique Du Gelidium Sesquipedale (Clem.) Thur. (Gélidiacées, Rhodophycées) Sur Les Côtes Du Massif Armoricain. Vegetatio 1967, 19, 8–10.
- Tsiamis, K.; Taşkin, E.; Orfanidis, S.; Stavrou, P.; Argyrou, M.; Panayotidis, P.; Tsioli, T.; Cicek, B.A.; Marcou, M.; Küpper, F.C. Checklist of Seaweeds of Cyprus (Mediterranean Sea). Bot. Mar. 2014, 57, 153–166.
- Azevedo Neto, A.I.; Parente, M.I.; Botelho, A.Z.; Prestes, A.C.L.; Resendes, R.; Afonso, P.; Alvaro, N.V.; Milla-Figueras, D.; Raul, R.M.; Tittley, I.; et al. Marine Algal Flora of Graciosa Island, Azores. Biodivers. Data J. 2020, 8, 1–29.
- Neto, A.I.; Cravo, D.C.; Haroun, R.T. Checklist of the Benthic Marine Plants of the Madeira Archipelago. Bot. Mar. 2001, 44, 391–414.
- Gabriel, D.; Fredericq, S. The Marine Macroalgae of Cabo Verde Archipelago: An Updated Checklist. Arquipélago Life Mar. Sci. 2019, 36, 39–60.
- John, D.M.; van Reine, W.P.; Lawson, G.W.; Kostermans, T.B.; Price, J.H. A Taxonomic and Geographical Catalogue of the Seaweeds of the Western Coast of Africa and Adjacent Islands. Beih. Zur Nova Hedwig. 2004, 127, 1–139.
- Adama, D.; Mohammed, A.; Maroua, H.; Mohammed, E.; Essalmani, H.; Mouna, D. Distribution and Biomass Assessment of Macroalgae from Moroccan Strait of Gibraltar. Acta Ecol. Sin. 2021, 41, 442–450.
- Bahammou, N.; Cherifi, O.; Bouamama, H.; Rezzoum, N.; Sabri, H.; Boundir, Y. Checklist of Rhodophyceae and the First Report of Aglaothamnion Tripinnatum and Gaillona Gallica in the Moroccan Coastline. Egypt. J. Aquat. Res. 2021, 47, 101–107.
- Akrong, M.O.; Anning, A.K.; Addico, G.N.D.; deGraft-Johnson, K.A.A.; Adu-Gyamfi, A.; Ale, M.; Meyer, A.S. Spatio-Temporal Variations in Seaweed Diversity and Abundance of Selected Coastal Areas in Ghana. Reg. Stud. Mar. Sci. 2021, 44, 101719.
- Mendoza-González, A.C.; Mateo-Cid, L.E.; García-López, D.Y. Inventory of Benthic Marine and Estuarine Algae and Cyanobacteria for Tabasco, México. Biota Neotrop. 2017, 17, e20170379.
- de Casamajor, M.N.; Lalanne, Y.; Derrien-Courtel, S.; Maria Gorostiaga, J.; le Gal, A.; Huguenin, L.; Quintano, E.; Lissardy, M. Cystoseira Baccata Meadows along the French Basque Coast (Bay of Biscay) as a Reference for the Implementation of the Water Framework and Marine Strategy EU Directives. Cont. Shelf Res. 2019, 182, 12–21.
- McHugh, D.J. A Guide to the Seaweed Industry; Technical Paper n. 441; Food & Agriculture Organization: Rome, Italy, 2003; ISBN 92-5-104958-0.
- Santelices, B. Patterns of Reproduction, Dispersal and Recruitment in Seaweeds. Oceanogr. Mar. Biol. Annu. Rev. 1990, 28, 177–276.
- mac Monagail, M.; Cornish, L.; Morrison, L.; Araújo, R.; Critchley, A.T. Sustainable Harvesting of Wild Seaweed Resources. Eur. J. Phycol. 2017, 52, 371–390.
- Verdura, J.; Sales, M.; Ballesteros, E.; Cefalì, M.E.; Cebrian, E. Restoration of a Canopy-Forming Alga Based on Recruitment Enhancement: Methods and Long-Term Success Assessment. Front. Plant Sci. 2018, 9, 1832.
- Carmona, R.; Vergara, J.J.; Lahaye, M.; Niell, F.X. Light Quality Affects Morphology and Polysaccharide Yield and Composition of Gelidium Sesquipedale. J. Appl. Phycol. 1998, 10, 323–331.
- Quintano, E.; Díez, I.; Muguerza, N.; Figueroa, F.L.; Gorostiaga, J.M. Bed Structure (Frond Bleaching, Density and Biomass) of the Red Alga Gelidium Corneum under Different Irradiance Levels. J. Sea Res. 2017, 130, 180–188.
- Alfonso, B.; Sansón, M.; Sangil, C.; Expósito, F.J.; Díaz, J.P.; Hernández, J.C. Herbarium Macroalgae Specimens Reveal a Rapid Reduction of Thallus Size and Reproductive Effort Related with Climate Change. Mar. Environ. Res. 2022, 174, 105546.
- Borja, Á. Cartografía y Evaluación de La Biomasa Del Alga Gelidium Sesquipedale En La Costa Guipuzcoana. Inv. Pesq. 1987, 51, 199–224.
- Borja, Á. Impacto de La Cosecha y Recuperación de La Biomasa Del Alga Gelidium Sesquipedale Sometida a Dos Formas de Explotación En El País Vasco (España). Aquat. Living Resour. 1994, 7, 59–66.
- Borja, A.; Chust, G.; Fontán, A.; Garmendia, J.M.; Uyarra, M.C. Long-Term Decline of the Canopy-Forming Algae Gelidium Corneum, Associated to Extreme Wave Events and Reduced Sunlight Hours, in the Southeastern Bay of Biscay. Estuar. Coast. Shelf Sci. 2018, 205, 152–160.
- Pascual, M.; Borja, A.; Eede, S.V.; Deneudt, K.; Vincx, M.; Galparsoro, I.; Legorburu, I. Marine Biological Valuation Mapping of the Basque Continental Shelf (Bay of Biscay), within the Context of Marine Spatial Planning. Estuar. Coast. Shelf Sci. 2011, 95, 186–198.
- Quintano, E.; Ganzedo, U.; Díez, I.; Figueroa, F.L.; Gorostiaga, J.M. Solar Radiation (PAR and UVA) and Water Temperature in Relation to Biochemical Performance of Gelidium Corneum (Gelidiales, Rhodophyta) in Subtidal Bottoms off the Basque Coast. J. Sea Res. 2013, 83, 47–55.
- Gorostiaga, J.M.; Santolaria, A.; Secilla, A.; Díez, I. Sublittoral Benthic Vegetation of the Eastern Basque Coast (N. Spain): Structure and Environmental Factors. Bot. Mar. 1998, 41, 455–465.
- Lee, R.E. Phycology, 5th ed.; Cambridge University Press: Cambridge, UK, 2018; ISBN 9781107555655.
- Seoane-Camba, J. Gelidium Sesquipedale (Clem) Thuret Cultivation in Galicia (Spain). Lagascalia 1997, 19, 179–186.
- Gaspar, R.; Pereira, L.; Sousa-Pinto, I. The Seaweed Resources of Portugal. Bot. Mar. 2019, 62, 499–525.
- Melo, R. Gelidium Commercial Exploitation: Natural Resources and Cultivation. J. Appl. Phycol. 1998, 10, 303–314.
- Lotze, H.K.; Milewski, I.; Fast, J.; Kay, L.; Worm, B. Ecosystem-Based Management of Seaweed Harvesting. Bot. Mar. 2019, 62, 395–409.
- Hossein Hoseinifar, S.; Chew, K.W.; Araújo, R.; Calderón, F.V.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Tasende, M.G.; Ghaderiardakani, F.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 626389.
- Veeragurunathan, V.; Vadodariya, N.; Chaudhary, J.P.; Gogda, A.; Saminathan, K.R.; Meena, R. Experimental Cultivation of Gelidium Pusillum in Open Sea along the South East Indian Coast. Indian J. Geo Mar. Sci. 2018, 47, 336–345.
- Melo, R.A.; Harger, B.W.W.; Neushul, M. Gelidium Cultivation in the Sea. Hydrobiologia 1991, 221, 91–106.
- Titlyanov, E.A.; Titlyanova, T.V.; Kadel, P.; Lüning, K. Obtaining Plantlets from Apical Meristem of the Red Alga Gelidium sp. J. Appl. Phycol. 2006, 18, 167–174.
- Wijayanto, A.; Widowati, I.; Winanto, T. Domestication of Red Seaweed (Gelidium latifolium) in Different Culture Media. J. Mar. Sci./Ilmu Kelaut. 2020, 25, 39–44.
- Boulus, A.; Spaneir, E.; Friedlander, M. Effect of Outdoor Conditions on Growth Rate and Chemical Composition of Gelidium Crinale in Culture. J. Appl. Phycol. 2007, 19, 471–478.
- Salinas, J.M.; Valdés, L. Influence of Temperature and Photoperiod on the Re-Attachment Process of Gelidium Sesquipedale. J. Appl. Phycol. 1993, 5, 317–326.
- Rojas, H.R.; León, M.N.; Rojas, O.R. Practical and Descriptive Techniques for Gelidium Rex (Gelidiales, Rhodophyta) Culture. Hydrobiologia 1996, 326/327, 367–370.
- Chiheb, H.; García-Jiménez, P.; Robaina, R.R.; Hassoun, M.; Riadi, H. Développement d’un Stock de Semences (Seedstocks) de l’algue Rouge Gelidium Corneum (Gelidiaceae, Rhodophyta). Eur. Sci. J. 2018, 14, 1857–7881.
- Duarte, P.; Ferreira, J.G. A Model for the Simulation of Macroalgal Population Dynamics and Productivity. Ecol. Model. 1997, 98, 199–214.
- Li, Y.; Liu, J.; Zhang, L.; Pang, T.; Qin, R. Effects of Temperature on the Photosynthetic Performance in Mature Thalli of the Red Alga Gelidium Amansii (Gelidiaceae). Aquaculture 2019, 512, 734320.
- Sousa-Pinto, I.; Murano, E.; Coelho, S.; Felga, A.; Pereira, R. The Effect of Light on Growth and Agar Content of Gelidium Pulchellum (Gelidiaceae, Rhodophyta) in Culture. Hydrobiologia 1999, 398–399, 329–338.
- Vergara, J.J.; Niell, F.X.; Torres, M. Culture of Gelidium Sesquipedale in a Chemostat System: Biomass Production and Metabolic Responses Affected by N Flow. J Appl Phycol 1993, 5, 405–415.
- Hurtado, A.Q. Genetic Resources for Farmed Seaweeds—Thematic Background Study; Food & Agriculture Org.: Rome, Italy, 2022.
This entry is offline, you can click here to edit this entry!
