1. Molecular Understanding of Amaryllidaceae Alkaloids Biosynthesis
Even though the pharmacological aspect of AAs has extensively been explored, the full understanding of the AA biosynthetic pathway and the characterization of enzymes responsible for catalyzing the different biosynthetic reactions demand more efforts. This knowledge would enable the establishment of improved systems or sustainable platforms for the production of these valuable biologically active compounds. Combined application of early labeling study followed by latest omics strategies have accelerated the discovery of AAs biosynthetic enzymes [
4,
75]. After the proposition of the biosynthetic route of different intermediates, several biosynthetic enzymes were predicted based on the nature of the biochemical reaction and by homology with enzymes involved in alkaloid biosynthesis of other plant families. Databases generated from transcriptomic and metabolic analysis of different species of Amaryllidaceae support the presence of different enzyme families involved in AAs pathway [
45,
54,
76,
77,
78,
79,
80,
81].
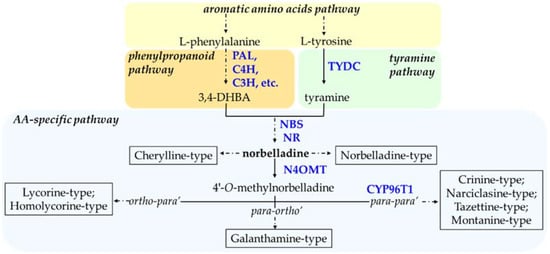
The AA biosynthetic pathway utilizes two common amino acids, namely
L-tyrosine and L-phenylalanine, as building blocks to produce a vast range of alkaloids with diverse biological activities. The first reactions of AA biosynthesis involve the formation of the ‘precursors’ from the phenylpropanoid and tyramine pathways (
Figure 2). As such,
L-tyrosine is decarboxylated by the enzyme tyrosine decarboxylase (TYDC) to yield tyramine while the production of the second building block, 3,4-dihydroxybenzaldehyde (3,4- DHBA), is achieved via the phenylpropanoid pathway by the action of enzymes such as phenylalanine ammonia-lyase (PAL), cinnamate 4-hydoxylase (C4H),
p-coumarate 3-hydroxylase (C3H), to name but a few. TYDC was characterized from
Lycoris radiata, a galanthamine producing Amaryllidaceae plant [
82]. The functional characterization of PAL and C4H in
L. radiata was reported using heterologous expression in bacteria [
77].
Figure 2. Biosynthetic routes to main types (boxed) of Amaryllidaceae alkaloid (AA). Arrows without labeling reflect chemical reactions where no enzyme was characterized. Enzymes that have been identified are labeled in blue. A solid arrow shows one enzymatic step, whereas a broken arrow symbolizes multiple enzymatic reactions. Following 4′O-methylnorbelladine, the regioselective phenol-phenol’ coupling reaction is indicated in the broken arrow, leading to various AA-types. Enzyme abbreviations: 3,4-DHBA, 3,4-dihydroxybenzaldehyde; PAL, phenylalanine ammonia-lyase; C4H, cinnamate 4-hydroxylase; C3H, coumarate 3-hydroxylase; TYDC, tyrosine decarboxylase; NBS, norbelladine synthase; NR, noroxomaritidine/norcraugsodine reductase; N4OMT, norbelladine 4′-O-methyltransferase; CYP96T1, cytochrome P450 monooxygenase 96T1.
Despite having a remarkable diversity in structure and biological activity, all AAs are derived from a common intermediate, norbelladine. The condensation of tyramine and 3,4-DHBA yields norbelladine and was shown to be catalyzed either by norbelladine synthase (NBS) or by noroxomaritidine/norcraugsodine reductase (NR), in both cases with low yield [
77,
83]. NBS was characterized from
N. pseudonarcisus king Alfred and
L. aestivum [
77]. GFP-tagged
LaNBS and CFP-tagged NR showed that both enzymes are localized to the cytosol, which suggests that the first committed step of AA biosynthesis probably occurs in the cytosol [
77,
84].
Norbelladine can either be utilized directly to generate norbelladine- and cherylline-type AAs or be further methylated by norbelladine 4′-
O-methyltransferase (N4OMT) to give 4′-
O-methylnorbelladine (
Figure 2). The structural feature of cherylline-type of AAs suggests the occurrence of 3′-
O-methylation during the biosynthesis of these types of AAs, although it remains to be proven. The specific synthesis of both 3′-
O-methylated and 4′-
O-methylated AAs suggest that regioselective methylation is important to determine the types of the end product of AAs biosynthesis route. The characterization of norbelladine OMT from
Narcissus sp.
aff. pseudonarsissus suggests that methylation by
NpN4OMT happens specifically at 4′-
O position of norbelladine [
78]. However, later studies on
L. radiata OMT (
LrOMT) propose that methylation can occur either in the 3′-
O or 4′-
O position of norbelladine, 3,4-DHBA, or caffeic acid. Kinetic study of
LrOMT indicates that it has a higher affinity for 3,4-DHBA as substrate compared to norbelladine. The methylated forms of 3,4-DHBA (i.e., vanillin and isovanillin) could also be condensed with tyramine to generate 3′ or 4′-
O-methylnorbelladine. However, up until now, none of the possible methylated forms of 3, 4-DHBA were tested as a substrate for NBS.
One step deeper in the AA pathway, and depending on the type of phenol-coupling reaction, the 4′-
O-methylnorbelladine can be directed to 1) galanthamine-type through
para-ortho’, 2) lycorine-type AAs by
ortho-para’, and 3) crinine-type of AAs by
para-para’ phenol coupling reactions (
Figure 2). These types of C-C phenol-coupling reactions are putatively catalyzed by members of the cytochrome P450 enzyme family. For example,
NpCYP96T1 was shown to catalyze the
para-para’ oxidative reaction of 4′-
O-methylnorbelladine into noroxomaritidine and was also shown to catalyzed formation of the
para-ortho’ phenol coupled product,
N-demethylnarwedine, as less than 1% of the total product [
85]. Aside from CYP96T1 and NR, there are no other steps (genes or enzymes) that have been identified in the formation of phenol-coupled AA-types to date (
Figure 2).
Plants synthesize specialized metabolites by using complex biosynthetic routes that derive from primary metabolic pathways. AAs biosynthesis is a multifaceted process that involves different regulatory elements and gene functions. The expression of certain genes involved in plant metabolism also changes with different climatic and environmental factors [
27]. Furthermore, it also varies within different developmental stage of plant [
45]. It remains challenging to correlate gene expression and metabolite accumulation
in planta, as the site of metabolite synthesis may differ from the site of accumulation. For example, nicotine biosynthesis occurs in the root of tobacco but accumulates in the aerial part of the plant [
86], whereas morphine biosynthesis starts in sieve elements of the phloem but accumulates in adjacent laticifers cells in opium poppy [
87]. As such, in vitro cultures have been an essential tool to decipher the alkaloid biosynthesis pathway. In 2011, Tahchy et al. used deuterium-labeled precursors fed to in vitro cultures of
L. aestivum. In this study, the authors followed the transfer of labeled precursor 4′-
O-methyl-d
3-norbelladine from media into shoot and then its metabolization into lycorine and galanthamine. This study demonstrated that 4′-
O-methylated-norbelladine was a key intermediate AAs [
88]. Until now, AAs specific genes such as
NBS, N4OMT, CYP96T1, and
NR have been characterized and confirmed from
Leucojum sp.,
Narcissus sp.,
Lycoris sp. cultures [
44,
45,
46,
47], however, our molecular understanding regarding this complex biosynthesis route of AAs and its regulation is still unclear. Furthermore, the pattern of relative expression of putative AAs biosynthetic genes (in fields versus in vitro and in differentiated versus undifferentiated tissues of
Narcissus development) added some clear knowledge regarding their role in alkaloid biosynthesis [
89]. A study performed on callus culture of
L. radiata showed how different factors, such as temperature (cold treatment), osmotic pressure (PEG treatment), or elicitor treatment (methyl jasmonate), can influence
LrOMT gene expression pattern [
90]. Thus, in vitro system cultures are a powerful tool to uncover AAs biosynthesis and gene regulation that should be thoroughly exploited.
2. Synthetic Biology for AA Biosynthesis
Although the complete biosynthetic pathway of AAs is not resolved, and up to now the AAs demand has not been sufficiently fulfilled by a plant source, a synthetic biological approach could be a powerful approach to produce AAs. Recent achievements in synthetic biological approaches include the production of complex biomolecules such as noscapine (a benzylisoquinoline alkaloid from opium poppy) and its halogenated derivatives (anticancer) in
Saccharomyces cerevisiae, assembling 30 biosynthetic enzymes from plant, bacteria, and mammal, with yeast itself including seven plant endoplasmic reticulum localized genes [
91]. This success gives hope for producing complex biomolecules such as AAs by using a synthetic biological approach.
Proper selection of host organism is the starting point of synthetic biological approach. The chosen organism should be producing (or easily modified to produce) enough core metabolites such as aromatic amino acids,
L-phenylalanine, and
L-tyrosine, precursors needed for the biosynthesis of target specialized metabolite such as AAs. Selection of host species will also rely on prior knowledge of their ease of engineering, established cloning tools, culture techniques, and possibly scaling up to industrial requirements. Due to rapid growth and easy handling, microbial hosts such as yeast (
Saccharomyces cerevisiae), and to a lesser extent
Escherichia coli, were used to produce plant-derived high value alkaloids like morphinan alkaloids [
92,
93,
94]. Furthermore, the production of aromatic amino acid (precursor for AAs) and associated upstream gene/enzyme were well studies in these hosts [
95]. Precursors such as
L-tyrosine and
p-coumaric have been already produced in
E. coli [
96,
97]. Recently, unicellular photosynthetic organisms such as microalgae and cyanobacteria became interesting research platforms because of their unicellular physiology, together with their photosynthetic, heterotrophic, and mixotrophic lifestyles [
98]. Moreover, plant-based genetic engineering technique is also emerging in model plants such as
Nicotiana tabacum and
N. benthamiana [
99].
Once a host organism is selected, availability of precursor molecules can be enhanced by modifications to its metabolic pathway, such as gene deletions, swapping of endogenous enzymes with more active homologues, or overexpression of endogenous metabolic genes. Then, a route to the desired specialized metabolites can be planned and implemented. A candidate pathway is first outlined through selection of stepwise chemical intermediates leading from host metabolism to the target compound, followed by selection of enzymes to carry out each specified reaction [
100,
101]. Even though the lack of knowledge in the AAs biosynthetic pathway hinders this approach as of yet, it could be partially overcome by creating libraries of gap-filling genes candidate generated from huge plant transcriptomic database, as available for thousands of plants or as part of the PhytoMetaSyn project [
102]. In addition, the decrease in the cost of DNA synthesis helps accelerate gene characterization from its native source and ultimately facilitate the production of complex biomolecules like AAs [
102,
103,
104]. Such work was done to produce polyketides. Soon, platform of synthetic approach will not only provide techniques to produce AAs but also help in the biosynthesis of novel AAs derivatives with improved biological and physiological properties. In example, once the complete identification of genes encoding enzymes required for the biosynthesis of galanthamine is achieved, one more enzyme could be added in the transgenic construct that could involve glycosylation, shifting the polarity of parent molecule, and eventually improving drug uptake by the human body.
This entry is adapted from the peer-reviewed paper 10.3390/biom12070893