Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biology
Gelidium corneum (Giant Gelidium or Atlantic agar) is a well-known red seaweed harvested for its high-quality agar content. Agar is a mixture of the polysaccharides used in the food industry as a gelling, thickener, clarifying, and stabilizer agent. The best agar quality is also used in the laboratory as bacteriological agar.
- harvested biomass
- waste biomass
- Gelidium corneum applications
- Gelidium life cycle
- Gelidium cultivation
1. Introduction
Seaweeds are marine macroscopic photosynthetic organisms classified into three taxa, according to their main accessory pigment: red seaweeds (Rhodophyta) present phycoerythrin, green seaweeds (Chlorophyta) contain chlorophyll b, and brown seaweeds (Phaeophyceae) exhibit fucoxanthin [1]. These organisms occur mainly in coastal areas, whether rocky or sandy shores, salt marshes, swamps, lagoons, estuaries, or coral reefs, but also in deep-water and open water floating communities, distributed in tropical, temperate, and polar regions. Zonation and community distribution depend on different physical and chemical parameters such as temperature, substrate, salinity, pH, nutrients availability, hydrodynamics, light, tides, wind, and pollution level. Biological factors such as epiphytism, herbivory, and disease also shape the distribution of seaweeds [2].
Seaweeds are known for their healthy primary and secondary metabolite content, such as a high protein content with a balanced essential amino acid content, low lipid content, and high fibre and mineral content. They also contain hydrocolloids, pigments, fatty acids, polyphenols, vitamins, minerals, and terpenoids, and other biomolecules [3,4,5,6,7]. These bioactive compounds show a beneficial effect on human health and well-being, such as antioxidant, antimicrobial, anti-cancer, anti-inflammatory, and antithrombotic effects, and immune-system improvement, among others [8,9,10,11,12,13]. However, besides human welfare, there are many new biotechnological applications, including in the cosmetics industry, as a moisturizer, anti-ageing, and UV protector [14,15,16,17], in agriculture, as a biostimulant or biofertilizer [18,19,20,21], and in bioremediation and biosorption, due to the seaweeds’ ability to remove contaminants from the surrounding water [22,23,24]. Seaweed extracts are also gaining research interest as a bioplastic product due to the nature of their polymers, producing resistant durable biofilms [25,26]. Another current approach is the use of seaweed waste biomass to produce biofuels (biogas, methane, or bioethanol) since this process does not increase the atmospheric net CO2 [27,28]. First-generation biofuels are derived from vegetable oil, starch, or sucrose, usually derived from crops. Second-generation biofuels are derived from lignocellulosic waste biomass. Third-generation biofuels are produced from algae. The second- and third-generation biofuels are much more land-use and environmentally efficient than first-generation biofuels [29,30]. These applications depend on the quantity and nature of the biomolecules present in algal biomass. Thus, one of the key challenges is to be able to efficiently extract these bioactive compounds. Making use of all the constituents of the seaweeds, while keeping their characteristics and bioactivities intact, requires sequential processing of all the material, and often the purification of the algal by-products [31]. The concept of a biorefinery has been proposed allowing the extraction and, therefore, use of all useful components of seaweed in a cascade process, cost-effectively, adopting a zero-waste approach while reducing the impact on climate change [32]. Furthermore, in a circular economy framework, any economic activity must have a positive impact on the environment, and the market encourages the recycling of products rather than extracting new resources. In this production and consumption model, all forms of waste are returned to the economy and/or used more efficiently, allowing the life cycle of products to be significantly extended [33]. Therefore, in a circular economy context, seaweed biomass waste should be processed into new products, and many authors are addressing this opportunity.
For many decades, one of the most industrially sought-after seaweeds was Gelidium. Gelidium sp. is a canopy-forming red seaweed (Rhodophyta) known for its high quality and content in agar [34,35,36,37,38]. Agar is a phycocolloid existing in the seaweed cell wall, consisting of a heterogeneous mixture of two polysaccharides, agarose, and agaropectin, the first with gelling properties and the second with thickening properties [39,40]. It is a semi-transparent, shiny, tasteless, odourless, and very hydrophilic colloid. Due to the formation of coiled helices, it forms very strong gels retaining water molecules when the agar solution is heated [41]. Gelidium sp. is the primary source of high-quality agar (high gelling strength and low sulphate content) and bacteriological grade agar, which is obtained only from this genus [2]. Although agar can be extracted from different species, such as Pterocladia, Pterocliadiella, Ahnfeltia, Acanthopeltis, and Gelidiella, the world agar market depends almost on wild-harvested Gelidium sp. and on cultivated Gracilaria sp., which produces a lower quality and lower price agar [42,43]. Currently, the most harvested agarophyte is the Gelidium corneum, being harvested in France, Italy, Portugal, Spain, and Morocco [44]. This wild harvest along with other human impacts, such as climate change, raises concern about the sustainability of the resource [45] and a global Gelidium landing shortage was recently diagnosed in 2018 [46]. Besides, the cultivation of Gelidium, although viable, did not reach enough yields to be economically profitable [47]. Hence, the management of the resource should be approached with attention.
Despite the importance of the agar market and the conservation concern, Gelidium sp. has been studied in the last two decades to evaluate other biomass properties such as antioxidant [48,49,50], antimicrobial [6,14,51,52], anti-inflammatory [53], antiproliferative or cytotoxic [54,55], biosorption of contaminants [56,57,58,59,60,61,62,63], and phyto-stimulant [64]. Besides, the use of agarophytic biomass has been proposed by several authors for biorefinery, including in the energy sector [65,66,67]. These properties disclose the potential use of G. corneum in several applications for which different types of biomasses may be used: harvested, stranded, and waste. The use of harvested biomass for other purposes than as an agarophyte competes with the agar industry and raises serious management and conservation concerns. Yet, the stranded biomass and waste may also be suitable to incorporate into new bio-based materials. The agar industry, e.g., produces annually many tonnes of residual Gelidium biomass, which are treated as waste [68] or are used in the fertilizer industry with a very low commercial value [6,69]. However, this waste biomass can have other uses, and, thus, a better valorisation, adding value to this biomass already used industrially, and diversifying its use. This may create new business opportunities for coastal populations, who are economically dependent on this valuable natural resource.
2. Gelidium corneum Biology, Distribution, and Ecology
Gelidium corneum common names: Atlantic agar, Giant Gelidium (English), Gelidium imperial (French), Ágar, Limo-encarnado, Cabelo-de-cão (Portuguese), Ocle, Caloca (Spanish) [70,71].
The Genus Gelidium includes, currently, 144 taxonomically accepted marine species [72] distributed worldwide. Among these species, Gelidium corneum (Hudson) J.V. Lamouroux (formerly Gelidium sesquipedale) is one of the best-known species. It is a cartilaginous dark-red seaweed (division Rhodophyta), with flattened branches with spoon-shaped branchlets, and creeping stolons at the base, up to 20 (30) centimetres tall, forming large tufts. Erect thalli grow from a system of creeping axes attached to rocky substrates through rhizoids (Figure 1) [69,70,73,74,75].

Figure 1. Macroscopic image of Gelidium corneum collected in Centre Portugal.
Gelidium is a clonal-modular seaweed [2]; consequently, it spreads laterally and vegetatively over the soil surface via creeping axes. It also produces erect thalli (fronds). Storms and grazing remove the fronds but not the creeping axes, which remain attached to the substrate. Regeneration and growth of erect fronds from the creeping axes are common and fast [76]. An interesting feature of Gelidium populations is the ability to grow from vegetative reproduction. These erect fronds can have an autonomous life when fragmented and can reattach to the substrate, so vegetative propagation, through fragmentation, is a frequent method of colonisation [76]. The species has a wide distribution, occurring in Atlantic Europe [6,73,77,78,79,80,81,82], Mediterranean Sea [83], Atlantic islands [84,85,86], Atlantic Africa [87,88,89,90], and Atlantic America [91]. Guiry also mentions populations in the Indic and Pacific oceans, namely in India, Indonesia, Korea, Vietnam, and Australia, but no published information on these locations could be found [72] (Figure 2).
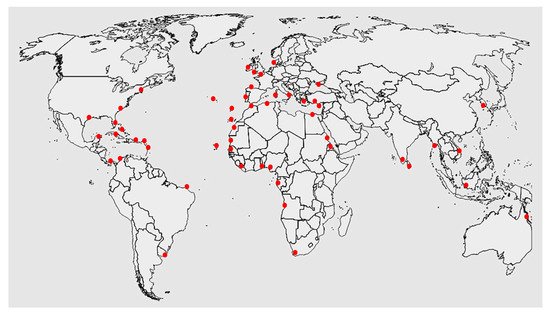
Figure 2. World distribution of Gelidium corneum (data from Guiry [72]).
G. corneum grows in temperate to tropical areas, with seasonal temperatures ranging between 10 and 25 °C, in partly shaded habitats, with strong tides and sea currents. In Europe, G. corneum forms widespread beds, usually subtidal zones up to about 25 m in depth. As to the substrate, the species prefers growing on slightly sloping regular bedrock, with little to moderate sand sedimentation [77,92]. In these temperate waters, Gelidium species reach high abundances and frequencies. Regardless of the species or the latitude, these are slow-growing organisms, up to 100 mm y−1 [76,93,94]. G. corneum is a canopy-forming seaweed, which is to say, it is a habitat-forming seaweed, creating a stable and complex community providing food, shelter, nursery, and habitat for many other species, such as invertebrates, fish, and other smaller algae [95,96]. G. corneum is sensitive to environmental parameters such as temperature, light, nutrients, and water movement [97]. Santelices [76] also states several biological factors affecting productivity, comprising morphology, age of the fronds, thallus part, reproductive state, seasonality, crop density, life history phase, and geographic and ecological origin of the species. Additional events of importance affecting Gelidium populations include extreme low tides, storms, and grazing [36,76].
In many areas of the globe, the cumulative impacts of human pressure, such as habitat destruction, pollution, over-harvesting, invasive species, and ocean warming, decrease the resilience of the seaweeds and promote the loss of the seaweed’s biomass. G. corneum is no exception, showing a decline in the past decades. In response to these disruptions, shifts in the distribution patterns of canopy species occur. Notably, these shifts are observed with a decline in canopy-forming species, the increase in morphologically simpler warm-water species and coralline algae, and the progressive introduction and expansion of non-indigenous species [45,78,79,98,99,100,101,102,103,104,105].
3. Gelidium corneum Life Cycle
The genus has a complex life cycle, representative of the most evolved red seaweeds (class Florideophyceae), with a triphasic isomorphic life cycle [74]. This life cycle is portrayed by a haploid independent gametophyte, producing gametes through mitosis (either male spermatangia or female carpogonia). The male gametes (spermatia) are released and pass to the trichogyne of the female gamete (carpogonium) where fertilization occurs (Figure 3). The mitotic division of the zygote produces gonimoblast filaments, the first diploid generation (carposporophyte phase), which grow within the female gametophytes. These carposporophytes produce carposporangia inside which diploid carpospores are formed by mitosis. Carpospores are released and each matures into an independent diploid tetrasporophyte, the second diploid generation. The tetrasporangial mother cell divides by meiosis to produce four haploid tetraspores, each becoming a new gametophyte [106]. Although isomorphic, the tetrasporangial are more common than the gametangial thalli. This finding suggests that tetrasporophytes are more robust and competitive and the gametophytes are more sensitive to environmental conditions and less viable [76].
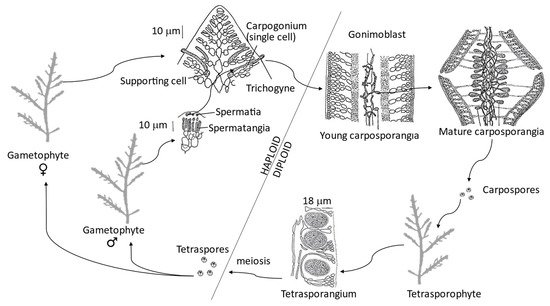
Figure 3. Triphasic isomorphic haplodiploid life cycle of Gelidium corneum, adapted from Lee [106].
The species is reported to have a certain inability to attach itself to a substrate through spore colonisation. So, sexual reproduction is often replaced by vegetative reproduction, in which the creeping thalli produce a disk of rhizoids that can attach and penetrate suitable substrates [107].
4. Gelidium Harvesting
Different species of Gelidium are harvested in the world to produce agar. G. corneum is harvested in Portugal, Spain, France, and Morocco, G. amansii and G. latifolium in Japan, Taiwan, Korea, and Indonesia, and G. robustum in Mexico. Smaller quantities of G. lingulatum, G. chilense, and G. rex are harvested in Chile, whereas G. pristoides, G. abbottiorum G. pteridifolium, G. capense are harvested in China, Namibia, and South Africa [46,92,108,109]. The biomass may be gathered from beach-cast seaweeds, using nets or rakes, a method common in countries such as South Africa, Australia, and New Zealand [110]. The attached thalli may also be cut by hand. More recently, seaweeds are harvested mechanically with the biomass being plucked off by divers, stowing the seaweeds in bags or baskets which are then lifted onto a boat [95,111]. When the creeping axes are removed, regeneration of the vertical thalli may take several years to regenerate, allowing the area to be invaded by other algae [94]. Harvesting, therefore, has a direct impact on the biomass and structure of seaweed beds and marine biodiversity. Harvesting canopy-forming seaweeds affects the morphology, canopy structure, thalli growth and regeneration, standing stock, and species composition of the foundation species. In turn, these changes affect the ecological roles of the canopy-forming seaweeds in marine ecosystems [110].
No doubt wild seaweed harvesting is facing the challenge of balancing the socio-economic and environmental sustainability of the activity. The impact on the wild stocks depends on the methods used, the mechanical clear-cutting being more severe than the hand-harvesting. For G. corneum, the creeping taxa must be left unharmed, allowing Gelidium populations to recover rapidly [94]. Over the past decades, management of natural resources has tackled the need to protect species and ecosystems, enabling habitat protection to sustain species diversity and abundance, whilst simultaneously granting sustainable exploitation of the marine resources. There is a need to evaluate permanent stocks of Gelidium where they are still harvested, to define harvest effort, to study the possibility of restocking, to establish and respect the harvest season, and to apply local resource management regulations [110].
5. Gelidium Cultivation
The demand for algal biomass for industrial purposes has far outstripped the capacity that traditional harvesting of wild stocks can provide. Trying to meet the demand, different attempts to cultivate Gelidium species have been performed for the past decades with some biological success but without economical relevance due to the slow growth of the genus [93]. These attempts were carried out in the laboratory, inland (tanks or ponds), or in the sea (net bag method, rafts, and net pouch), forming spores, fragments, and grown from reattachment thalli [74,107,112,113,114,115,116,117,118,119]. Different cultivation conditions were tested, assessing the influence of temperature, season, irradiance, nutrients, and water movement productivity of the biomass [94,117,120,121,122,123]. Although the experiments succeeded to a greater or lesser extent, they resulted in low yields. Yields vary among species, but cultivation points to a maximum yield of 25 kg FW m−2, which cannot compete economically with wild harvesting [47]. The only species that has been industrially produced is G. amansii in ponds, recorded for North Korea, but with very little data available [46,124].
Despite these setbacks, G. corneum is an important biological resource with multiple applications, so the feedstock cannot depend exclusively on wild resources. Further scientific research is, therefore, required to develop more efficient cultivation techniques that will produce abundant quality biomass at a competitive price compared to wild biomass. Therefore, to introduce economically profitable cultivation of Gelidium, it will be required to work on genetic improvement of the genus, through the selection of the more productive strains or genetic engineering [47]. Genetic engineering, together with further studies on cultivation and reproduction techniques, will hopefully allow full domestication of Gelidium, as has already been achieved for other seaweed species in the past years.
This entry is adapted from the peer-reviewed paper 10.3390/earth3030045
This entry is offline, you can click here to edit this entry!
