Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell & Tissue Engineering
Mammalian cell lines are preferred for the production of functional, complex recombinant proteins including mAbs, with Chinese hamster ovary (CHO) cells being used in most instances. Despite significant advances in cell growth control for biologics manufacturing, cellular responses to environmental changes need to be understood in order to further improve productivity. Metabolomics offers a promising approach for developing suitable strategies to unlock the full potential of cellular production.
- immunotherapy
- monoclonal antibodies
- metabolomics
- industrial production
- process optimization
1. Introduction
In 1986, Muromumab became the first FDA-approved mAb for the prevention of kidney transplant rejection. Since 1990, and especially since the approval of the first fully human antibody in 2004, the number of mAbs available in the pharmacopeia has increased. Biotherapeutics have been used for the treatment of a variety of medical conditions including cancers, organ transplants and autoimmune, cardiovascular, respiratory and neurological diseases, which is witnessed by the steady increase in the number of FDA-approved treatments [1]. The pharmaceutical industry has made great progress in developing reliable and efficient bioproduction processes that meet the demand for new biotherapeutics including monoclonal antibodies (mAbs). Sixty-eight new mAbs were approved between 2014 and 2018, thus representing more than 50% of all new biopharmaceutical products on the market, and mAbs represented the most lucrative product class, with a revenue of $123 billion registered in 2017 [2].
mAbs are complex structures and require proper folding, assembly and post-translational modifications, such as glycosylation, to ensure their functionality and efficacy. Unlike microbial systems that are limited in post-translational modifications, mammalian cell lines are apt to generate diverse functional recombinant glycoproteins, with Chinese Hamster Ovary (CHO) cells being used for the production of 84% of FDA-approved biotherapeutics in 2018 [2].
The culture of CHO cells in bioreactors is a tightly regulated process that has seen significant advances since the 90s [3]. The steps involved in the development of a cell culture production process include the design and the selection of a stable protein-secreting cell line, the optimization of media and culture operating conditions at a small scale using medium- to high-throughput screening methods and finally the upscaling of the process, which must comply with good manufacturing practices (GMPs) [4,5]. Despite major advances in cell culture processes and the optimization of cell culture media, the cellular response to changes in the culture environment and the subsequent impact on cell growth and productivity needs to be better understood in order to fully optimize the production of biologics.
In this regard, multidimensional ‘omics’ approaches are powerful tools used to improve the knowledge and to design suitable strategies for unlocking the full potential of CHO cells. Genomics, proteomics, transcriptomics and metabolomics are recent and complementary fields of study that have been instrumental in deciphering biological mechanisms influencing cell growth in bioreactors. Metabolomics is a promising approach in the bioproduction field, as it detects the downstream products of the other ‘omics’ sciences (genomics, transcriptomics and proteomics for the characterization of DNA, RNA and proteins/enzymes, respectively) and is believed to accurately mirror the cellular phenotype. Metabolomics involves the intracellular and extracellular quantification of small molecules called metabolites [6], concentrations of which strongly vary as a function of cellular responses to environmental changes [7]. The standardized preparation of the samples, be it debris-free culture supernatants or washed cells, is the first step of metabolomic analysis. This is followed by the metabolic profiling of the samples either by nuclear magnetic resonance (NMR) or mass spectrometry (MS). A bioinformatic analysis then connects metabolites to known metabolic pathways and quantifies metabolic fluxes [8,9]. Two distinct methodologies called untargeted and targeted metabolomics are widely used for this purpose. Untargeted metabolomics is an unbiased analysis measuring all detectable metabolites present in the sample and can enable the discovery of new molecules impacting cell metabolism. Targeted metabolomics focuses on groups of known metabolites that are chemically defined. Both methods are complementary and are often used in combination to gain maximum insights [10]. Metabolomics analysis can also be coupled with the metabolic labeling of the cell substrate by adding non-radioactive isotope tracers (usually synthetic metabolites labelled with carbon-13, 13C) to the culture media prior to mass spectrometry analysis. This combined approach allows for metabolic flux analysis (MFA) and the calculation of reaction rates as well as the identification of precursor–product relationships among metabolites [11].
2. The Exponential Growth Phase: High Nutrient Uptake Enabling Cell Growth
The exponential phase is characterized by a steep increase in the viable cell density (VCD) in the cell culture. This growth is possible because nutrients are available in the culture medium, from which (1) catabolism generates energy and (2) various substrates are used for biomass generation. Both steps will be described in this section, and the main metabolic trends are summarized in Figure 1.
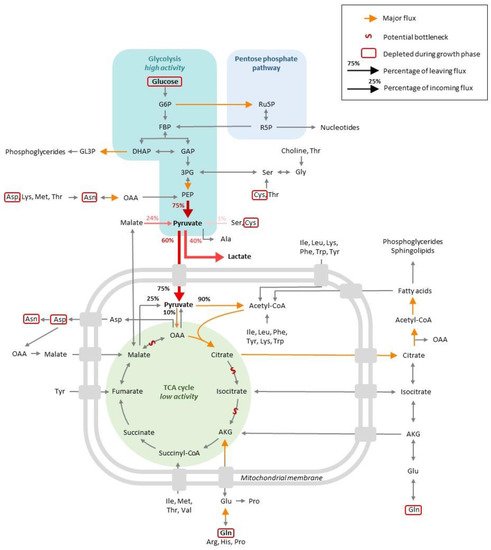
Figure 1. Global view of the central CHO cell metabolism at the exponential phase of growth.
2.1. Carbon Source Catabolism Generates Energy for the Cells as Nutrients Are Largely Available (Step 1)
MFA studies have shown that glucose contributes to 70% of the total carbon influx, while pyruvate from medium serves as an additional carbon source at the beginning of the culture, with 80% of it being consumed in the first day of culture [18,19]. Similarly, another study concluded that glucose represents 65% of the entering carbon, with 10% coming from glutamine and 25% from other amino acids [23]. A lower contribution was also reported (40–50%) [24,25], although this can partly be explained by the fact that glucose consumption can vary simply due to different culture conditions, specifically, in the present case, culture volumes [26]. Besides these subtle differences, publications overall agree on the fact that glucose constitutes the main carbon source for growth.
Glucose taken up by cells can be phosphorylated and supplied to the glycolysis pathway for ATP production or to the pentose phosphate pathway (PPP), which contributes to redox homeostasis and biosynthesis. Numerous studies report that during the exponential phase, glucose is mainly oxidized via glycolysis, which results in the formation of pyruvate. A significant portion of the pyruvate formed is converted into lactate, which is secreted and acidifies the medium, resulting in growth inhibition, with the rest being used to supply the TCA cycle [11,16,19,23,24,26,27,28,29,30,31,32,33,34,35,36,37]. This metabolic state was previously recognized as the Warburg effect and characterizes cancer cells that consume high levels of glucose. Based on the potential energy load of glucose, it can be considered that, in the sense, the carbon flux is “wasted” by the cells, in that it is not targeted to the TCA and yields high lactate levels instead. This lowers the production of the C4-6 precursors necessary for biomass generation [38,39].
It has been estimated that 75% of glucose consumption goes toward lactate production in cultures of adherent and suspension-growing CHO cells [11,33,40]. In perfusion cultures, however, a more balanced proportion has been measured, with 55% of the pyruvate being estimated to generate lactate and 45% entering the TCA cycle [32]. On the contrary, MFA studies on batch/fed-batch cultures report that 25–40% of cytosolic pyruvate is converted into lactate by the action of lactate dehydrogenase, an effect that is observed for both CHO WT cells and protein-producing CHO derivatives [18,23,41], while 50–60% of the pyruvate pool is channeled into the TCA cycle. These discrepancies might be attributed to different media compositions. For instance, it has been shown that glutamine provided in the media can strongly influence glucose and pyruvate metabolism [42]. It was also shown that the overexpression of bcl2, an anti-apoptotic gene, can influence the proportion of pyruvate entering the TCA and, as a result, lactate formation [34]. A number of studies also analyzed the differences between high and low producer clones. Certain studies revealed that (i) high producers possess higher levels of intracellular NADH, suggesting a higher level of glycolysis in combination with the TCA cycle and/or oxidative phosphorylation [43], and (ii) some differences exist in the timing of glucose consumption and lactate formation between high and low producer cell lines, with high producers consuming lactate earlier in the culture compared to low producers [19]. Only minor differences were reported in another study, suggesting that further investigations are needed to clarify how productivity might be correlated to the early phases of glucose metabolism [44].
At the pyruvate branch point, it was estimated that about 75% of pyruvate comes from glycolysis, while 25% results from the conversion of malate by the malic enzyme and a minor fraction from the degradation of amino acids such as serine or cysteine [40,41,42]. A total of 90% of pyruvate flux would enter in the TCA following its conversion to acetyl-coA, with the remaining 10% entering the TCA cycle after conversion into oxaloacetate by pyruvate carboxylase [32,33,42].
At the late exponential phase, a decrease by one-third in terms of glucose consumption and glycolytic activity was measured in favor of lactate uptake [18,37]. Interestingly, it was possible to prolong the exponential phase by the adding pyruvate and amino acids to the medium [16].
The pentose phosphate pathway (PPP) represents an alternative route of glucose oxidation that regenerates the NADPH that contributes to the redox balance in the cytoplasm and C5 sugars that are involved in biosynthetic reactions, in particular nucleotide biosynthesis. Pentose phosphate pathway (PPP) activity has been characterized during the exponential phase, and would appear that its contribution to the overall carbon flux depends on the experimental models. In the perfusion mode, the proportion of glucose entering the PPP was estimated at 20–40% [32], while studies conducted in batch/fed-batch conditions reported a very low or negligible contribution from glucose to this pathway [11,33,34,40]. This is in contradiction with one study conducted on fed-batch cultures, which reported that 80% of glucose was engaged in the PPP pathway, accounting for most of the cytosolic NADPH generation during this growth phase [23]. Thus, these studies reported major differences in terms of glucose utilization between glycolysis and PPP, perhaps reflecting variations in the composition of media, including in terms of glutamine levels [42], medium supply in fed-batch cultures, specific clones or yields in the production of recombinant proteins.
Glycolysis oxidative reactions fed by glucose or PPP intermediate species result in the generation of pyruvate that can enter the TCA cycle as acetyl-CoA. As mentioned above, TCA cycle fluxes are low during the exponential phase, with a large portion of pyruvate being used for lactate production [11,13,17,34]. As a possibility, any overabundant glucose available during initial cell culture might be used for energy maintenance through lactate production rather than for the TCA cycle and oxidative phosphorylation. It was also hypothesized that the TCA cycle might be impaired in transformed CHO cell lines due to a reduced capacity to convert citrate into α-ketoglutarate (AKG) [16,45]. Another bottleneck might exist between malate and oxaloacetate due to a limited enzyme capacity, which would result in limited malate-aspartate shuttling and, hence, NAD regeneration in the cytosol. In addition, this limitation in oxaloacetate supply prevents the incorporation of mitochondrial pyruvate into the TCA cycle, resulting in the conversion of pyruvate conversion into lactate in the cytoplasm [44]. Although lactate production is significant, as mentioned earlier, the TCA is active, with the main carbon source coming from glucose oxidation to pyruvate and the rest from glutamine and essential amino acids [23]. Interestingly, it has been shown that the secretion of TCA intermediates (succinate, malate, citrate and fumarate) represents approximately the same level of TCA cycle flux during the exponential growth and stationary phases, possibly indicating that the TCA cycle is operating at close to maximal enzymatic capacity throughout cell culture [44]. However, this conclusion was reached upon using 1H-NMR, which is comparatively less sensitive than MS. It has also been suggested that the culture scale impacts the metabolism of cells. Cells growing in production-scale bioreactors may rely more on glycolysis for energy production than cells growing at lab scale that synthesize more TCA cycle intermediates [46].
Regarding nucleotides and nucleosides at the exponential phase, little information is available in the literature. Up to 90% of ATP production has been suggested to result from the TCA cycle [41], with a downward trend along the exponential phase [17]. While ADP and AMP concentrations were found to be similar in the cytosol and mitochondria, ATP concentration was higher in the cytosol than in the mitochondria, suggesting that the transport of ATP out of the mitochondria might render ATP formation thermodynamically efficient [30]. At this phase of growth, when comparing high and low producer clones, steady-state ATP concentrations are comparable, but the production and consumption rates correlate with productivity in Escherichia coli and CHO cells [43]. The addition of adenosine monophosphate (AMP) or guanosine monophosphate (GMP) to the culture media led to a 3-fold induction of caspase activity, the highest observed for all metabolites tested, contrasting with the addition of cytidine monophosphate (CMP) and uridine monophosphate (UMP), which had no effects [47]. This pro-apoptotic effect was also reported in other studies on CHO cells [48] and IEC-6 intestinal epithelial cells [49]. This means that, even in the presence of nutrients and in the absence of high levels of ammonia and lactate, toxic metabolites can cause cell death.
On average, 25% of the essential amino acids consumed were not supplied for biomass or IgG production during exponential growth and instead generated TCA cycle intermediates [44]. This interpretation is supported by a second study in which the rate of glutamine uptake during the early exponential phase greatly exceeded the biosynthetic demand for biomass or antibody production, which reflects its use in catabolic energy production [18].
2.2. The Use of Energy Enables Biomass Production (Step 2)
To the researchers' knowledge, no metabolomics studies focused on nucleic acid and protein synthesis. This section will focus on lipid metabolism, which is extensively covered by metabolomics studies.
One group of studies measured an increase in intracellular concentrations of medium and long chain fatty acids in line with the need for cell growth during the exponential phase. In line with this observation, the contribution of fatty acid oxidation to the acetyl-CoA mitochondrial pool was found to be negligible during the exponential phase [11], with acetyl-CoA being mainly directed toward fatty acid synthesis at this phase of cell growth [23]. A large fraction of citrate was found to be transported to the cytosol for its conversion into oxaloacetate and acetyl-CoA [31,33]. An MFA study calculated that about 25% of the citrate synthase flux is channeled via citrate lyase to the biosynthesis of fatty acids. In this study, the TCA cycle intermediates mostly fueled lipid synthesis and rendered negligible amounts of amino acids [32]. A study performed on adherent CHO cells demonstrated that acetyl-CoA produced from glutamine does not contribute significantly to fatty acid biosynthesis, suggesting that other sources of carbon such as glucose do [33]. As a component of the glycerol-based phospholipids that compose cell membranes, glycerol was also found to accumulate extracellularly, representing 5% of the carbon engaged in glycolysis [26,44]. In their review, Pereira et al. (2018) stated that glycerol released into the medium results in a loss of carbon to the cells. For the authors, glycerol can be regarded as a storage compound or redox sink in the regeneration of NAD pools [38,39]. Other studies have shown that choline, choline phosphate, ethanolamine phosphate, cytidine-diphosphate-choline (CDP) and CDP-ethanolamine are almost depleted during the exponential phase, while other precursors of the cell membrane constituents are accumulated [12,17]. It was suggested by Selvarasu et al. that this might be a reflection of poor membrane lipid metabolism regulation in CHO cells and that this might trigger the transition from the exponential to the stationary phase [17]. In support of this hypothesis, an increase in extracellular phosphocholine and glycerol-3-phosphocholine was reported and correlated with intracellular caspase activity and apoptosis [44,47]. A thorough investigation of the altered metabolic flux, including in the glycerophospholipid metabolic pathway, might help in the design of strategies to enhance cell culture viability.
3. The Stationary Phase: Stabilized Growth and High Recombinant Protein Production
3.1. Alternative Carbon Source Catabolism Enables Energy Production and Cell Maintenance
As a general trend, most of the carbon and nitrogen sources were found to be consumed at lower rates during the stationary phase compared to the exponential phase [34,40,44]. Thus, a 5-fold decrease in glycolytic activity was reported to be accompanied by an equivalent reduction in glutaminolysis and AKG production [33]. The stationary phase also witnesses a major shift in the metabolism of lactate, which is not so much produced but consumed, enabling TCA cycle replenishment, as the glucose concentration decreases [11,16,17,18,31,33,34,44,56]. Glucose consumption continues, even though at a decreased rate, until it is fully depleted [11,27,41,44]. One report, namely [18], indicated a slight decrease in glutaminolysis, while glucose fluxes do not drop below 50% of their initial rate. A decrease in glucose consumption and glycolysis fluxes by one-third was observed at the late exponential phase and the stationary phase [18]. These key characteristics were also observed for antibody-producing GS-NS0 mouse myeloma cells. In such cells, lactate was consumed earlier when proliferation was artificially stopped, which also correlated with increased productivity [28]. Evidence was provided that glucose is not depleted during the stationary phase but at the entry in the decline phase [16]. The depletion of asparagine and aspartate was reported to occur at the entry into the stationary phase, well before the exhaustion of glucose. Moreover, pyruvate was identified as a growth-limiting metabolite, meaning that its depletion marks the transition from the exponential to the stationary phase [44]. The depletion of pyruvate was confirmed in a more recent study [31].
PPP fluxes increase during the stationary phase compared to the exponential phase by a factor of 5 to 6-fold, corresponding to 30% of the glucose uptake rate [11,33] or possibly even more [13,18,34]. Sengupta et al. hypothesized that during the stationary phase, cells might experience more oxidative stress, leading to a higher utilization of the PPP to generate NADPH for reductive reactions. This is supported by a study on Escherichia coli that showed that the bacteria fail to fight oxidative stress during the stationary phase because of the downregulation of genes involved in aerobic electron transport [57]. One recent report showed that the downregulation of the PPP, as opposed to its activation, occurred during the stationary phase in CHO cells [31], but this relationship has not been extensively studied in CHO cells and deserves further investigation.
Most studies agree that succinate, malate, citrate and fumarate accumulate during the stationary phase in the culture supernatant [12,20,27,46,58]. At this stage, carbon influx into the TCA cycle is provided by glucose (50%), glutamine (40%) and other amino acids (10%) entering the TCA cycle in the form of acetyl-CoA or anaplerotic substrates including malate, α-ketoglutarate or oxaloacetate [41]. More importantly, a switch in pyruvate utilization is observed from lactate production and secretion to its translocation into the mitochondria and use in the TCA cycle, which reaches its peak activity [13,18,34]. Such TCA cycle activation may be secondary to exacerbated oxidative stress, resulting in a decreased NADH/NAD+ ratio and the consequent counter regulation of the regeneration of the NADH pool.
In addition to the publications that report the increased activity of the TCA cycle in the stationary phase, equivalent or decreased activity has also been reported. For example, MFA performed on adherent CHO cells during the stationary phase led to the conclusion that most of the pyruvate is diverted to the TCA cycle (instead of yielding lactate), with no impact on the TCA cycle, which operates at similar levels as during the exponential phase [11,33]. This was also observed in a study using 1H-NMR, where the secretion of succinate, malate, citrate and fumarate were found to represent the same proportion of the TCA cycle flux as during the exponential phase. This indicates that in these models, the TCA is operating at close-to-maximum enzymatic capacity throughout the culture time [44]. In other studies, the activities of glutamate dehydrogenase and pyruvate carboxylase, key anaplerotic enzymes, were found to be reduced 2-fold, and the flux from α-ketoglutarate to succinyl-CoA was also lowered to 50% [11,33]. Such decreased TCA cycle activity has also been observed in other works [16,17] and led to the conclusion that glucose might be redirected to other metabolic pathways to enable ATP generation and NADPH oxidation in the stationary phase, during which proliferation is limited and productivity is increased.
3.2. The Use of Energy Enables Recombinant Protein Production
The production of recombinant proteins by CHO cells ramps up during the stationary phase, accounting for 15% of incoming carbon fluxes, in contrast to only 3% during the early exponential phase [18].
In contrast to the exponential growth phase, about 25% of pyruvate formation results from lactate consumption during the stationary phase, with other minor sources being malate and serine together with pyruvate uptake from the media [33,40,44]. The entry of pyruvate into the TCA cycle by conversion into acetyl-CoA was measured to be similar in the exponential and stationary phases. On the contrary, its conversion into oxaloacetate via pyruvate carboxylase activity was found to be undetectable during the stationary phase [33].
Regarding glycerophospholipids, one study reported that the transition between exponential growth and the stationary phase is marked by the intracellular appearance and then accumulation in media of glycerol-3-phosphate and glycerol, the latter being presumably synthesized from glycerol-3-phosphate [14,16]. Carinhas et al. estimated that 25% of the carbon engaged in glycolysis ends up in glycerol [44]. The surge in glycerol concentration observable at the transition from the exponential to the stationary phase was interpreted to reflect the lipid biosynthesis that is required for cell proliferation-associated membrane formation, the turnover of membrane lipids as well as the secretion vesicles used for protein excretion [14]. An increase of glycerophosphocholine, a product of the degradation of phosphatidylcholine, which is a major component of the plasma membrane, was also observed. This was interpreted to reflect cell membrane degradation and cell growth limitation [17]. Surprisingly, fatty acid biosynthesis was found to be as high during the stationary phase as during the exponential phase, suggesting another role in addition to cell growth that was not elucidated by the authors [33]. This is consistent with the hypothesis that lipid synthesis might reflect the need for membrane lipid turnover (plasma membrane and intracellular membranes involved in vesicular trafficking).
4. The Decline Phase: Media Exhaustion Resulting in Cell Death
Relatively few studies have investigated CHO metabolism during the decline of cell cultures. The final phase was found to be accompanied by the exhaustion of glucose, with a consequent decrease in glycolytic intermediates [16]. Pyruvate is depleted, while lactate remains relatively constant in batch culture [14]. In fed-batch [14], and HiPDOG (hi-end pH delivery of glucose) culture conditions [18], lactate utilization was observed during the decline phase. In this latter study, PPP flux was maintained even when cell density began to decline.
Regarding the TCA, Matuszczyk et al. have demonstrated the depletion of cytosolic pyruvate while mitochondrial pyruvate was available at higher concentrations, suggesting high TCA cycle activity [30]. This is in line with another study reporting that TCA cycle activity is even higher during the decline phase than during the early exponential phase [18]. On the contrary, a significant decrease in the metabolites of the TCA cycle compared to the other culture phases was reported in one study, and this was interpreted to be due to less carbon skeleton from glucose fueling the TCA during this phase [16]. This discrepancy may be explained by the fact that the former study was focused on an early phase of decline while the latter was focused on a terminal phase of decline and medium exhaustion.
Regarding nucleotide metabolism, ATP, ADP and AMP concentrations decreased with the culture time until the decline phase, but their distributions across the cytoplasmic and mitochondrial compartments differed. The ATP concentration was found to be higher in the cytosol than in the mitochondria during this culture phase, unlike in the cases of ADP and AMP, which were found at similar concentrations in both compartments [30].
In addition to a comprehensive understanding of cell metabolism during the decline phase, which needs further investigation, several strategies have been suggested to extend the productive phase of the cell suspension. For example, one study recommended the growing of cells in galactose-containing medium, as it is metabolized when glucose is depleted, which enables the maintenance of cell viability [59]. Another study suggested the addition of an anti-apoptotic agent to avoid excessive cell death [46].
This entry is adapted from the peer-reviewed paper 10.3390/cells11121929
This entry is offline, you can click here to edit this entry!
