Metal-organic frameworks (MOFs) are a type of crystalline porous material having organic ligands connected to metal clusters. This type of material shows a terrific design adaptability due to the almost unlimited combinations of metallic salts and organic ligands. Several scientists have been attracted by this important part of reticular chemistry, probably due to the fact that the researcher’s inventiveness is the only limitation in this research field. Thus, different research groups have greatly contributed to the exponential growth of this area, using these materials in a diverse range of applications, including catalysis, water harvesting, biomedicine and sensing.
- metal-organic frameworks
- light
- photoresponsive materials
- reticular chemistry
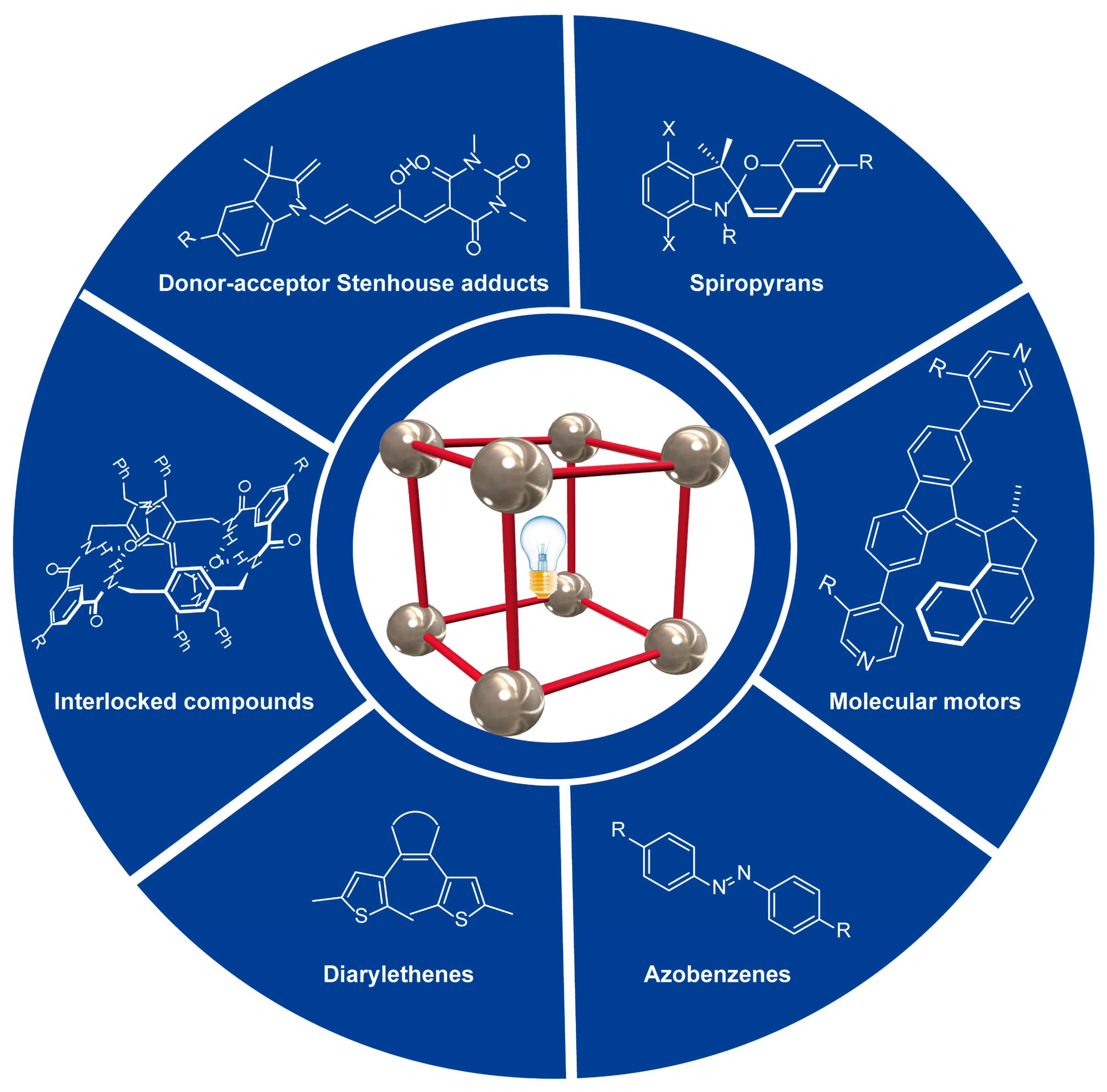
This entry is adapted from the peer-reviewed paper 10.3390/ijms23137121
References
- Helal, A.; Yamani, Z.H.; Cordova, K.E.; Yaghi, O.M. Multivariate metal-organic frameworks. Nat. Sci. Rev. 2017, 4, 296–298.
- Burtch, N.C.; Heinen, J.; Bennett, T.D.; Dubbeldam, D.; Allendorf, M.D. Mechanical properties in metal-organic frameworks: Emerging opportunities and challenges for device functionality and technological applications. Adv. Mater. 2017, 30, 1704124.
- Masoomi, M.Y.; Morsali, A.; Dhakshinamoorthy, A.; Garcia, H. Mixed-metal MOFs: Unique opportunities in metal-organic framework (MOF) functionality and design. Angew. Chem. Int. Ed. 2019, 131, 15330–15347.
- Belver, C.; Bedia, J. Metal organic frameworks for advanced applications. Catalysts 2021, 11, 648.
- Nargarkar, S.S.; Desai, A.V.; Ghosh, S.K. Stimulus-responsive metal-organic frameworks. Chem. Asian. J. 2014, 9, 2358–2376.
- Liu, Z.; Zhang, L.; Sun, D. Stimuli-responsive structural changes in metal-organic frameworks. Chem. Commun. 2020, 56, 9416–9432.
- Li, C.; Wang, K.; Li, J.; Zhang, Q. Recent progress in stimulus-responsive two-dimensional metal-organic frameworks. ACS Mater. Lett. 2020, 2, 779–797.
- Wei, M.; Wan, Y.; Zhang, X. Metal-organic frameworks-based stimuli-responsive polymers. J. Compos. Sci. 2021, 5, 101.
- Chattopadhyay, K.; Mandal, M.; Maiti, D.K. Smart metal-organic frameworks for biotechnological applications: A minireview. ACS Appl. Bio. Mater. 2021, 4, 8159–8171.
- Yan, D.; Wang, Z.; Zhang, Z. Stimuli-responsive crystalline smart materials: From rational design and fabrication to applications. Acc. Chem. Res. 2022, 55, 1047–1058.
- Zhang, X.; Chen, Z.; Liu, X.; Hanna, S.L.; Wang, X.; Taheri-Ledari, R.; Maleki, A.; Li, P.; Farha, O.K. A historical overview of the activation and porosity of metal-organic frameworks. Chem. Soc. Rev. 2020, 49, 7406–7427.
- Ji, Z.; Wang, H.; Canossa, S.; Wuttke, S.; Yaghi, O.M. Pore chemistry of metal-organic frameworks. Adv. Funct. Mater. 2020, 30, 2000238.
- Zhang, X.; Yu, T.; Au, V.K.-M. Photoresponsive metal-organic frameworks: Tailorable platforms of photoswitches for advanced functions. ChemNanoMat 2022, 8, e202100486.
- Jones, C.L.; Tansell, A.J.; Easun, T.L. The lighter side of MOFs: Structurally photoresponsive metal-organic frameworks. J. Mater. Chem. A 2016, 4, 6714–6723.
- Castellanos, S.; Kapteijn, F.; Gascon, J. Photoswitchable metal organic frameworks: Turn on the lights and close the windows. CrystEngComm 2016, 18, 4006–4012.
- Haldar, R.; Heinke, L.; Wöll, C. Advanced photoresponsive materials using the metal-organic framework approach. Adv. Mater. 2020, 32, 1905227.
- Bandara, H.M.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825.
- Cheng, H.-B.; Zhang, S.; Bai, E.; Cao, X.; Wang, J.; Qi, J.; Liu, J.; Zhao, J.; Zhang, L.; Yoon, J. Future-oriented advanced diarylethene photoswitches: From molecular design to spontaneous assembly systems. Adv. Mater. 2022, 34, 2108289.
- Kortekaas, L.; Browne, W.R. The evolution of spiropyran: Fundamentals and progress of an extraordinary versatile photochrome. Chem. Soc. Rev. 2019, 48, 3406–3424.
- Duan, Y.; Zhao, H.; Xiong, C.; Mao, L.; Wang, D.; Zheng, Y. Learning from spiropyrans: How to make further developments of donor-acceptor Stenhouse adducts. Chin. J. Chem. 2020, 39, 985–998.
- Pooler, D.R.S.; Lubbe, A.S.; Crespi, S.; Feringa, B.L. Designing light-driven rotary molecular motors. Chem. Sci. 2021, 12, 14964–14986.
- Gatti, F.G.; Leigh, D.A.; Nepogodiev, S.A.; Slawin, A.M.Z.; Teat, S.J.; Wong, J.K.Y. Stiff, and sticky in the right places: The dramatic influence of preorganizing guest binding sites on the hydrogen bond-directed assembly of rotaxanes. J. Am. Chem. Soc. 2001, 123, 5983–5989.
- Geng, J.; Mei, L.; Liang, Y.; Yuan, L.; Yu, J.; Hu, K.; Yuan, L.; Feng, W.; Chai, Z.; Shi, W. Controllable photomechanical bending of metal-organic rotaxane crystals facilitated by regioselective confined-space photodimerization. Nat. Commun. 2022, 13, 2030.
- Jiang, Y.; Tan, P.; Qi, S.-C.; Liu, X.-Q.; Yan, J.-H.; Fan, F.; Sun, L.-B. Metal-organic frameworks with target-specific active sites switched by photoresponsive motifs: Efficient adsorbents for tailorable CO2 capture. Angew. Chem. Int. Ed. 2019, 58, 6600–6604.
- Geng, J.; Liu, K.; Liang, Y.; Yu, J.; Hu, K.; Yuang, L.-H.; Feng, W.; Chai, Z.; Mei, L.; Shi, W. An azobenzene-modified photoresponsive thorium-organic framework: Monitoring and quantitative analysis of reversible trans-cis photoisomerization. Inorg. Chem. 2021, 60, 8519–8529.
- Sato, H.; Matsui, T.; Chen, Z.; Pirillo, J.; Hijikata, Y.; Aida, T. Photochemically crushable and regenerative metal-organic framework. J. Am. Chem. Soc. 2020, 142, 14069–14073.
- Fan, C.B.; Liu, Z.Q.; Gong, L.L.; Zheng, A.M.; Zhang, L.; Yan, C.S.; Wu, H.Q.; Feng, X.F.; Luo, F. Photoswitching adsorption selectivity in a diarylethene-azobenzene MOF. Chem. Commun. 2017, 53, 763–766.
- Williams, D.E.; Martin, C.R.; Dolgopolova, E.A.; Swifton, A.; Godfrey, D.C.; Ejegbavwo, O.A.; Pellechia, P.J.; Smith, M.D.; Shustova, N.B. Flipping the switch: Fast photoisomerization in a confined environment. J. Am. Chem. Soc. 2018, 140, 7611–7622.
- Martin, C.R.; Leith, G.A.; Kittikhunnatham, P.; Park, K.C.; Ejegbavwo, O.A.; Mathur, A.; Callahan, C.R.; Desmond, S.L.; Keener, M.R.; Ahmed, F.; et al. Heteromettalic actinide-containing photoresponsive metal-organic frameworks: Dynamic and static tuning of electronic properties. Angew. Chem. Int. Ed. 2021, 60, 8072–8080.
- Shepherd, N.D.; Wang, T.; Ding, B.; Beves, J.E.; D’Alessandro, D.M. Visible light stimulated bistable photo-switching in defect engineered metal-organic frameworks. Inorg. Chem. 2021, 60, 11706–11710.
- Danowski, W.; van Leeuwen, T.; Abdolahzadeh, S.; Roke, D.; Browne, W.R.; Wezenberg, S.J.; Feringa, B.L. Unidirectional rotary motion in a metal-organic framework. Nat. Nanotechnol. 2019, 14, 488–494.
- Danowski, W.; Castiglioni, F.; Sardjan, A.S.; Krause, S.; Pfeifer, L.; Roke, D.; Comotti, A.; Browne, W.R.; Feringa, B.L. Visible-light-driven rotation of molecular motors in a dual-function metal-organic framework enabled by energy transfer. J. Am. Chem. Soc. 2020, 142, 9048–9056.
- Saura-Sanmartin, A.; Martinez-Cuezva, A.; Bautista, D.; Marzari, M.R.B.; Martins, M.A.P.; Alajarin, M.; Berna, J. Copper-linked rotaxanes for the building of photoresponsive metal organic frameworks with controlled cargo delivery. J. Am. Chem. Soc. 2020, 142, 13442–13449.
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674.
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444.
