Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The skin has a multifactorial aging process, caused by both intrinsic and extrinsic factors. A major theory of aging involves cellular senescence or apoptosis resulting from oxidative damage as the skin’s antioxidant system tends to weaken with age. The human microbiota is a complex ecosystem that is made up of microorganisms (bacteria, fungi, and viruses). Both gut and skin microbiota have essential roles in the protection against invading pathogens, mediating inflammatory conditions, and the modulation of the immune system which is involved in both innate and adaptive immune responses.
- aging
- microbiota
- microbiome
- mycobiome
1. Human Microbiomes
Table 1 summarizes the differences between human gastrointestinal tract (GIT) microbiota and skin microbiota communities. The human GIT houses a complex ecosystem that is made up of trillions of microorganisms such as bacteria, fungi, and viruses, referred to as the gut microbiota [1]. The gut microbiome works to maintain host health and homeostasis, mediate inflammatory conditions, and modulate the immune system through a delicate balance of commensal and pathogenic bacteria [2][3]. However, the gut microbiome may be changed depending on lifestyle, nutrition, bacterial infections, antibiotics, surgical interventions, frailty, and inflammation [4][5][6]. An alteration of the intestinal bacteria results in microbial dysbiosis. A state of dysbiosis is characterized by a reduction in bacterial species diversity and a decrease in beneficial bacteria. Microbial variation can potentially affect the function of the microbiome by increasing intestinal permeability while compromising the absorption of nutrition, food metabolization, and immune system regulation [1][7][8][9]. A disruption of the intestinal microflora and its associated consequences can influence the pathology of various diseases, including aging [10][11].
Table 1. Differences between human gut microbiota and skin microbiota communities.
| Comparators | Gut Microbiota | Skin Microbiota | Citation |
|---|---|---|---|
| Microbial biomass |
|
|
[12][13] |
| Initial colonization pattern |
|
|
[14][15] |
| Microbial distribution |
|
|
[13][16] |
| Colonization stability |
|
|
[13][17][18][19] |
| Microbiota community |
|
|
[13][16][20] |
Abbreviation: GIT; gastrointestinal tract.
Skin is the largest organ in the human body. Similar to the gut microbiome, the skin microbiota is also composed of millions of microorganisms, including bacteria, fungi, and viruses. Some of these are beneficial symbiotics with essential roles in the protective barrier, preventing the invasion of pathogens. When an imbalance of commensals and pathogens occurs, skin disease or systemic disease can occur. Microorganisms reside at different depths or sub-compartments of the skin. Some microorganisms are variably present at the surface compared with deeper skin layers. Therefore, the capture of skin microbiota usually depends on the method used to sample these organisms. Most skin microbiome surveys have used amplicon sequencing. Recently, major technical and analytical breakthroughs have, however, enabled shotgun metagenomic sequencing studies. Additional studies with more invasive sampling techniques are necessary to fully understand the distribution of microorganisms in the skin. The overview of age-associated changes in gut and skin microbiota is demonstrated in Figure 2.
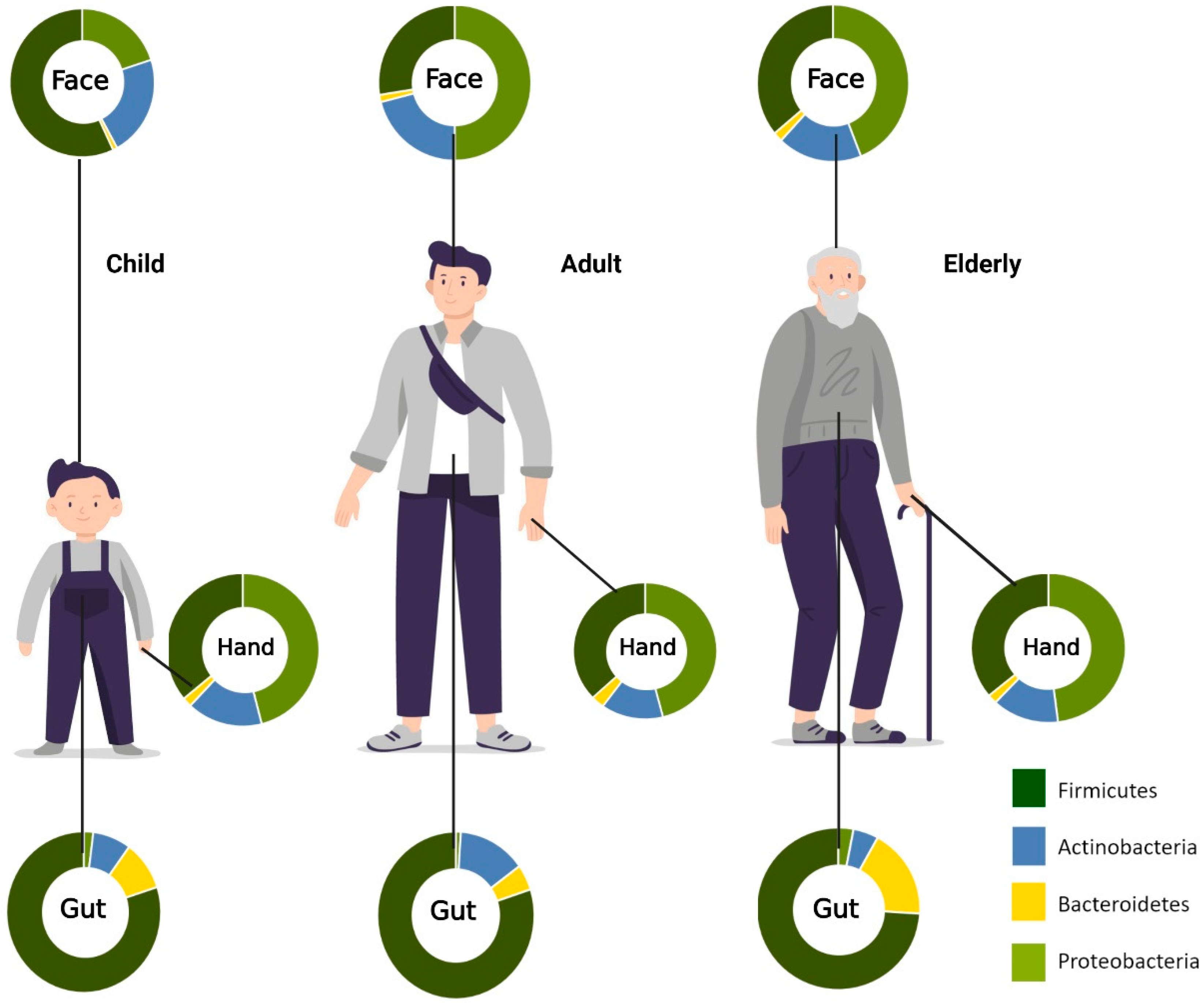
Figure 2. Aging-associated changes in skin and gut microbiota composition. Relative abundances of phyla on the face, hand, and gut. In the facial area, Firmicutes are most abundant in childhood, while Proteobacteria is most prominent in adulthood. For the hand microbiota, Proteobacteria was found to be the most predominant phylum from childhood to old age. Firmicutes, on the other hand, are most abundant in the gut across all age groups. (Created with BioRender.com) (accessed on 20 May 2022).
2. Skin Aging and Gut Microbiome
During the transition from adulthood to old age, the gut microbiota undergoes significant alterations. When compared to adults, there is a decline in microbial diversity and a greater inter-individual variation in microbiota composition in old people (>65 years old) [21]. It has also been demonstrated that microbiome composition can influence the rate of aging [6][22]. There is no known chronological threshold or age at which the microbiota composition abruptly changes; rather, these changes gradually occur over time [23]. The distinct microbial composition in the GIT has been attributed to aging and age-associated inflammation. For instance, a decline in the anti-inflammatory bacterial species was found in aged mice [24]. As shown in prior studies focusing on gut microbiota in centenarians, longevity is positively associated with an abundance of short-chain fatty acid (SCFA) producers, such as Clostridium cluster XIVa, Ruminococcaceae, Akkermansia, and Christensenellaceae [25][26]. The average phyla composition of centenarians was different from those of other elderly and adults. Furthermore, a recent study reported 116 microbial genes significantly correlated with aging, which were identified as a signature for longevity [27]. More diverse phyla were detected in the microbiota of centenarians compared to other groups. Additionally, good immunological and metabolic health-related bacteria, such as Akkermansia, Christensenellaceae, and Lactobacillus, were higher in centenarians than in other groups [28]. Correspondingly, the loss of Lactobacillus and Faecalibacterium, as well as the abundance of Oscillibacter and Alistipes genera along with the Eubacteriaceae family is linked to frailty in elderly people. Frail elderly people also have more proinflammatory Bacteroidetes commensals [21][29]. Controversially, some studies have shown centenarians’ microbiotas are less diverse than those of adult persons with decreased levels of Bifidobacterium, Bacteroides, and Enterobacteriaceae, and increased Clostridium spp. levels [25][30]. Several authors have suggested that the aging-associated differences in gut microbiota generally may not always be caused by aging, but they might be linked to a general decline in health status. According to age-related changes in microbiome diversity, recent findings have shown that a loss of diversity in the core microbiota groups is associated with aging-associated frailty rather than chronological age [21][23][31][32].
The production of a broad range of bioactive metabolites is a critical component of the gut’s function, serving as the most likely linkages between the gut microbiota and the host. SCFAs, such as butyrate, propionate, and acetate, are products of fiber fermentation by the gut microbiota and have been shown to exert anti-inflammatory and immunomodulatory effects [33][34][35][36]. A prior study demonstrated a dramatic decrease in the Firmicutes phyla and an increase in the Bacteriodetes phyla occurred from adulthood to old age, resulting in a decline in the Firmicutes-to-Bacteriodetes (F/B) ratio [37]. In particular, the F/B ratio is crucial for the production of SCFAs [38]. Generally, age-related dysbiosis can enhance the progression of aging, inflammation, and frailty, while compromising overall health and longevity.
Investigators have begun to explore the relationship between senescence and microbial dysbiosis. A recent study investigating the microbial composition in senescent models was performed [39]. In aged mice, the gut microbiome signatures associated with the markers of cellular senescence and inflammatory factors, known as senescence-associated secretory phenotype (SASP) were evaluated. Findings revealed that Clostridiales, Staphylococcus, and Lachnospiraceae positively correlated with all of the cellular senescence and inflammatory markers. Conversely, Coriobacteriaceae and Akkermansia correlated negatively with these markers. The relation between cellular senescence and microbial composition implies that microbial dysbiosis is involved in senescence. In addition, prebiotics and probiotics are efficient in preventing particular pathological conditions in elderly populations by suppressing inappropriate chronic inflammation and improving adaptive immune responses, thereby counteracting immunosenescence [40][41].
Furthermore, gut dysbiosis with age results in a leakage of proinflammatory microbial products via impaired intestinal permeability [29]. These products are then translocated into the bloodstream, leading to systemic effects. Microbial metabolites promote SASP damage through the upregulation of various inflammatory molecules, including tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), IL-1, IL-6, matrix metalloproteinases (MMPs), and others, contributing to the chronic proinflammatory state or inflammaging. As a consequence of dysbiosis, inflammaging and deficient immune surveillance thereby impair the removal of senescent cells.
3. Gut-Skin Axis and Skin Aging
The gut-skin axis describes the bidirectional communication pathway between the gut microbiome and the integumentary system via its immunological and metabolic properties (Figure 3). Bacterial microbes and their metabolites that enter blood circulation can travel through the body and affect distant tissue organs and the skin [42][43]. Although it is difficult to ascertain a causal relationship between the gut microbiome and skin conditions, multiple studies indicate a link between them with several dermatological diseases being associated with gastrointestinal disorders and vice versa [44][45]. In addition, previous studies have demonstrated that the increased intestinal permeability caused by dysbiosis has led to an accumulation of bacterial metabolites in the skin, as well as impairment in epidermal differentiation and skin integrity [46][47]. The exact mechanism underlying gut-skin microbial interactions has not yet been fully elucidated. However, recent reports demonstrate oral probiotics to be beneficial in improving several signs of skin aging, including acidic skin pH, oxidative stress, photodamage, and skin barrier dysfunction [48]. Furthermore, studies determining the connection between Lactobacillus plantarum HY7714, Bifidobacterium breve B-3, and skin protection have been conducted. Findings suggest that there were functional substances in the skin–gut axis communication, which interact in a photoprotective manner, resulting in an anti-aging effect in a mouse model [49][50][51], and administration of Lactobacillus plantarum HY7714 can decrease the symptoms of UV-induced skin photo-aging in humans [52].
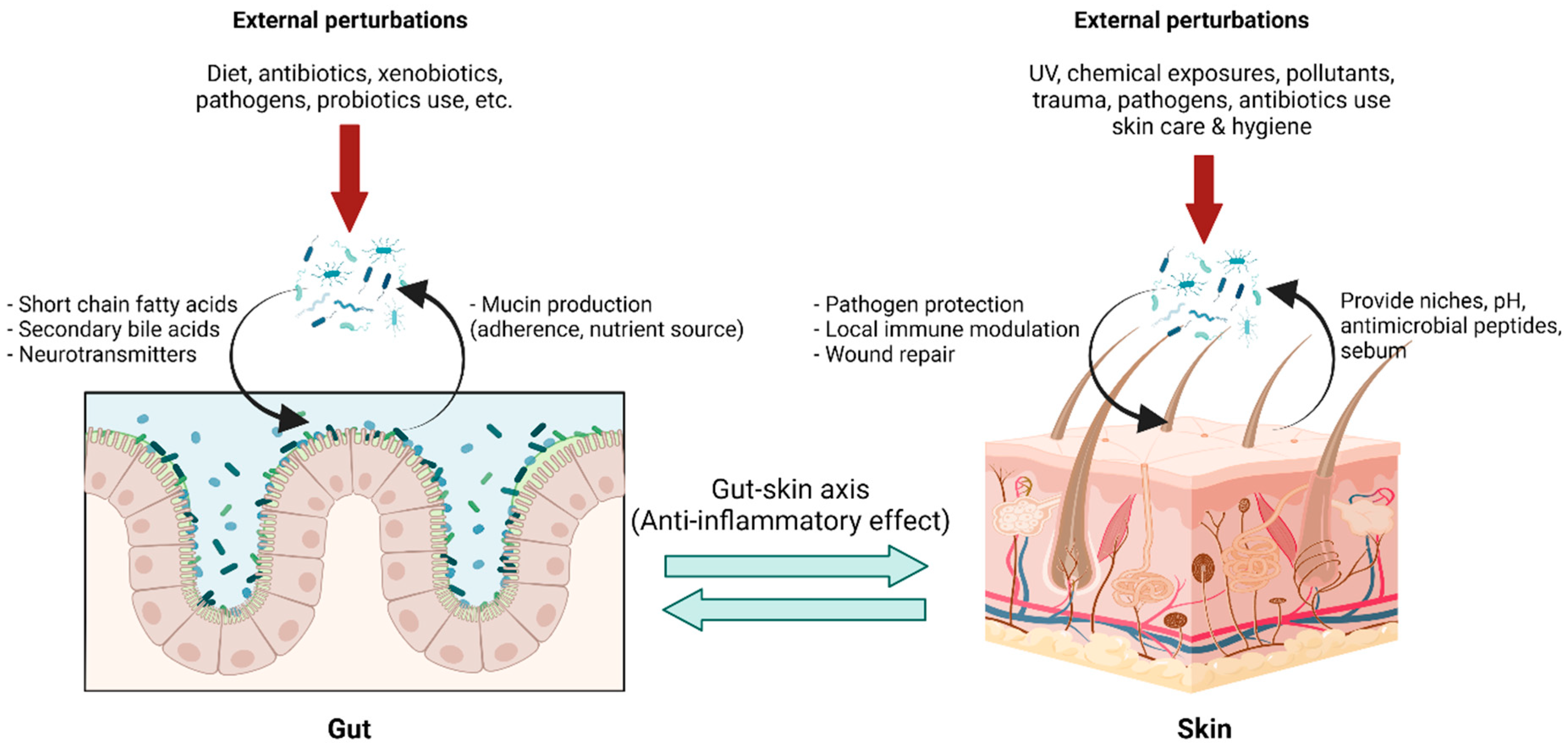
Figure 3. Gut-skin axis in a homeostatic state: The gut environment provides niches for gut microbiota with nutrients and optimal growth conditions, while gut microbiota carry out pleiotropic functions in maintaining body homeostasis. Microbial metabolites, for example, short-chain fatty acids, secondary bile acid, and several small molecules, not only locally maintain enterocyte functions but also exert systemic effects, including immune tolerance. This effect is linked to the skin via blood circulation, forming the so-called “gut-skin axis,” and provides an anti-inflammatory environment in the skin, optimizing interactions with skin microbiota. Skin microbiota in a homeostatic state help prevent pathogen colonization, modulating local immune responses and facilitating wound healing. Transient external perturbations, either cutaneous or mucosal, could disrupt microbiota composition, resulting in a transient dysbiosis state. The balanced microbiota could be recovered by perturbation removal, growth promotion of microbiota by proper diets, and, although in development, intervention with pro-and prebiotics. (Created with BioRender.com) (accessed on 12 May 2022). Abbreviation: pH, potential of hydrogen; UV, ultraviolet.
Gut dysbiosis (Figure 4), the impairment of senescent cell removal, and the accumulation of SASP factors can affect the function and integrity of the skin, leading to premature aging phenotypes. Notably, the upregulation of MMPs, which belong to SASP, is a contributory factor to age-related skin changes. MMPs reconstruct the extracellular matrix (ECM) by degrading proteins including collagen, fibronectin, elastin, and proteoglycans. The alterations made to the ECM by MMPs can influence skin wrinkling, sagging, and laxity [53][54]. However, the underlying mechanism addressing the relationship between the gut microbiome and skin aging characteristics has not yet been well established. Further studies are needed to advance the understanding of relationships between microbial composition, metabolite alteration, as well as accumulation, and skin phenotype changes.

Figure 4. Age-related intestinal dysbiosis: Age-related intestinal dysbiosis is generally characterized by a decrease in short-chain fatty acid producers, for example, Clostridiales, and Bifidobacterium, and enrichment of pro-inflammatory Proteobacteria including the opportunistic Enterobacteriaceae. It is likely to be a result of aging gut mucosa and external factors, for example, drug use, diet, and behavioral changes. Gut dysbiosis leads to a state of “leaky gut,” described by increased permeability of the gut mucosa due to tight junction disruption, allowing a small but periodic translocation of bacterial contents into the systemic circulation. Bacterial antigen, especially lipopolysaccharide, is pro-inflammatory, increasing circulatory pro-inflammatory cytokines, for example, TNFα, IL-1β, and IL-6. Chronic exposures to pro-inflammatory bacterial antigens have been hypothesized to contribute to, in addition to the aging process, the accumulation of cellular senescence and immunosenescence, both of which lead to a state of chronic low-grade systemic inflammation called inflammaging. Inflammaging was thought to be the basis of age-related aberrant conditions, including the immune dysregulation of the skin, which consequently leads to skin microbiota dysbiosis. Skin dysbiosis is associated with several dermatological diseases, with a higher proportion of pathogen colonizers and pro-inflammatory microbiota. (Created with BioRender.com) (accessed on 18 April 2022). Abbreviation: IL, interleukin; LPS, lipopolysaccharide; TNF, tumor necrotic factor.
4. Skin Aging and Skin Microbiomes
The skin microbiome plays a significant role in maintaining skin homeostasis and contributes to the skin’s barrier function to protect against the environment and potential pathogens [55]. Commensal bacteria compete for nutrients and space, inhibiting the reproduction of competitors via the production of antimicrobial compound peptides (AMPs), leading to inhibition against pathogen growth [55][56]. Skin microbes secrete enzymes involved in skin homeostasis; protease enzymes play a role in stratum corneum renewal, lipase enzyme is involved in lipidic film surface breakdown; and urease enzyme is implicated in urea degradation. Other roles of microbiota include the production of bacteriocin, quorum sensing, biofilms, and pH regulation by sebum and free fatty acid production [20]. In addition, the interaction between host tissue and microbiome resulted in the complex signals involved in innate and adaptive immune responses [57].
Age-related skin changes are attributed to combinations of internal factors (genetics and gender), environmental factors (pollution, sun exposure, and climate), and lifestyle factors (exercise, stress, sleep, nutrition, and skincare routine) [20][57][58]. Skin aging is characterized by a decrease in sebum, sweat, and immune function, resulting in significant alternations in the skin surface’s physiology, including lipid composition, sebum secretion, and pH. These affect skin dryness, collagen fragmentation, reduction in the total amount of collagen and elastin, as well as influencing the skin ecology, possibly shaping the skin microbiome [20][57]. Dimitriu, et al. studied bacterial microbiomes of 495 North American participants at four skin sites and the oral mucosa using 16s rRNA gene amplicon sequencing and found that demographics, lifestyle factors, physiology, and aging contribute to skin microbiota variations while the influence of ethnicity was the strongest association with the oral microbiomes [58].
Aging-related alteration of skin microbiome diversity has been described in several studies. Higher bacterial alpha diversity has been reported in older adults. A Japanese cohort study reported the difference in bacterial species between younger adults aged 21–37 years old and older adults aged 60–76 years old with skin site dependency. The showed a significant increase in Corynebacterium on the cheeks and forehead and Acinetobacter on the scalp in the older group. In contrast, Cutibacterium decreased in the cheeks, forehead, and forearms [59]. A study in North America also found that aging is associated with an increased abundance of Corynbacterial taxa, including C. kroppenstedtiin and C. amycolatum in the forehead area [58]. Juge, et al. studied the changing of microbiota diversity in Western European women, revealing a higher alpha diversity on older skin than on younger. The taxonomic composition analysis showed a decrease in Acinetobacter and an increase in Proteobacteria on older skin. At the genus level, old-aged skin exhibited an increase in Corynebacterium and a decrease in Cutibacterium relative abundance [60]. In another study, Somboonna, et al. studied the skin microbiota in 30 healthy Thai females aged 19–57 years and found Firmicutes was the most abundant bacterium in healthy elderly adults and acne-prone young adults. In contrast, Gemmatimonadetes, Planctomycetes, and Nitrospirae are more prevalent in healthy teenagers [61]. Howard et al. investigated the skin microbiome in the Caucasian women aged 20–70 years, reporting an age-related decrease in the sebocyte gland area and an increase in the natural moisturizing factors (NMF), skin lipids, and antimicrobial peptides (AMPs), resulting in a decrease in the relative abundance of Cutibacterium and Lactobacillus at the face, forearms, and buttocks in the older age group [62].
Modifying skin physiology during the aging process, such as hydration, sebum secretion, pH, and lipid composition, could predict changes in microbiota. Mukherjee et al. studied the relationship between the facial skin microbiome and variations in sebum and hydration levels in healthy female volunteers and revealed an increase in cheek sebum increased the relative abundance of Actinobacteria and Cutibacterium whereas microbiome diversity decreased [63]. Moreover, cutaneous immunity is weakened with age, thus further impairing the skin barrier and increasing skin infections and cancer susceptibility. Skin aging altered the immune cell composition with reduced Langerhans cells decreased antigen-specific immunity and increased Foxp3+ regulatory T cells [57].
This entry is adapted from the peer-reviewed paper 10.3390/life12070936
References
- Das, B.; Nair, G.B. Homeostasis and dysbiosis of the gut microbiome in health and disease. J. Biosci. 2019, 44, 1–8.
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836.
- Konturek, P.C.; Haziri, D.; Brzozowski, T.; Hess, T.; Heyman, S.; Kwiecień, S.; Konturek, S.J.; Koziel, J. Emerging role of fecal microbiota therapy in the treatment of gastrointestinal and extra-gastrointestinal diseases. J. Physiol. Pharmacol. 2015, 66, 483–491.
- Rodríguez, J.M.; Murphy, K.; Stanton, C.; Ross, R.P.; Kober, O.I.; Juge, N.; Avershina, E.; Rudi, K.; Narbad, A.; Jenmalm, M.C.; et al. The composition of the gut microbiota throughout life, with an emphasis on early life. Microbes Ecol. Health Dis. 2015, 26, 26050.
- Kashtanova, D.A.; Popenko, A.S.; Tkacheva, O.N.; Tyakht, A.; Alexeev, D.G.; Boytsov, S. Association between the gut microbiota and diet: Fetal life, early childhood, and further life. Nutrition 2016, 32, 620–627.
- Candela, M.; Biagi, E.; Brigidi, P.; O’Toole, P.; De Vos, W.M. Maintenance of a healthy trajectory of the intestinal microbiome during aging: A dietary approach. Mech. Ageing Dev. 2014, 136, 70–75.
- Belizário, J.E.; Faintuch, J.; Garay-Malpartida, H.M. Gut Microbiome Dysbiosis and Immunometabolism: New Frontiers for Treatment of Metabolic Diseases. Mediat. Inflamm. 2018, 2018, 1–12.
- Bischoff, S.C. Microbiota and aging. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 26–30.
- Biagi, E.; Franceschi, C.; Rampelli, S.; Severgnini, M.; Ostan, R.; Turroni, S.; Consolandi, C.; Quercia, S.; Scurti, M.; Monti, D.; et al. Gut Microbiota and Extreme Longevity. Curr. Biol. 2016, 26, 1480–1485.
- Boyajian, J.L.; Ghebretatios, M.; Schaly, S.; Islam, P.; Prakash, S. Microbiome and Human Aging: Probiotic and Prebiotic Potentials in Longevity, Skin Health and Cellular Senescence. Nutrients 2021, 13, 4550.
- Lakshminarayanan, B.; Stanton, C.; O’Toole, P.W.; Ross, R. Compositional dynamics of the human intestinal microbiota with aging: Implications for health. J. Nutr. Health Aging 2014, 18, 773–786.
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; NISC Comparative Sequencing Program; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; et al. Topographical and Temporal Diversity of the Human Skin Microbiome. Science 2009, 324, 1190–1192.
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155.
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975.
- Mueller, N.; Bakacs, E.; Combellick, J.; Grigoryan, Z.; Dominguez-Bello, M.G. The infant microbiome development: Mom matters. Trends Mol. Med. 2015, 21, 109–117.
- Dieterich, W.; Schink, M.; Zopf, Y. Microbiota in the Gastrointestinal Tract. Med. Sci. 2018, 6, 116.
- Faith, J.J.; Guruge, J.L.; Charbonneau, M.; Subramanian, S.; Seedorf, H.; Goodman, A.L.; Clemente, J.C.; Knight, R.; Heath, A.C.; Leibel, R.L.; et al. The Long-Term Stability of the Human Gut Microbiota. Science 2013, 341, 1237439.
- Jo, J.-H.; Deming, C.; Kennedy, E.A.; Conlan, S.; Polley, E.C.; Ng, W.-I.; Segre, J.A.; Kong, H.H.; NISC Comparative Sequencing Program. Diverse Human Skin Fungal Communities in Children Converge in Adulthood. J. Investig. Dermatol. 2016, 136, 2356–2363.
- Jo, J.-H.; Kennedy, E.A.; Kong, H.H. Topographical and physiological differences of the skin mycobiome in health and disease. Virulence 2016, 8, 324–333.
- Boxberger, M.; Cenizo, V.; Cassir, N.; La Scola, B. Challenges in exploring and manipulating the human skin microbiome. Microbiome 2021, 9, 1–14.
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184.
- Saraswati, S.; Sitaraman, R. Aging and the human gut microbiota—From correlation to causality. Front. Microbiol. 2015, 5, 764.
- O’Toole, P.W.; Jeffery, I.B. Gut microbiota and aging. Science 2015, 350, 1214–1215.
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P.; et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 2017, 21, 455–466.
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through Ageing, and Beyond: Gut Microbiota and Inflammatory Status in Seniors and Centenarians. PLoS ONE 2010, 5, e10667.
- Kong, F.; Hua, Y.; Zeng, B.; Ning, R.; Li, Y.; Zhao, J. Gut microbiota signatures of longevity. Curr. Biol. 2016, 26, R832–R833.
- Rampelli, S.; Candela, M.; Turroni, S.; Biagi, E.; Collino, S.; Franceschi, C.; O’Toole, P.W.; Brigidi, P. Functional metagenomic profiling of intestinal microbiome in extreme ageing. Aging 2013, 5, 902–912.
- Kim, B.-S.; Choi, C.W.; Shin, H.; Jin, S.-P.; Bae, J.-S.; Han, M.; Seo, E.Y.; Chun, J.; Chung, J.H. Comparison of the Gut Microbiota of Centenarians in Longevity Villages of South Korea with Those of Other Age Groups. J. Microbiol. Biotechnol. 2019, 29, 429–440.
- Biragyn, A.; Ferrucci, L. Gut dysbiosis: A potential link between increased cancer risk in ageing and inflammaging. Lancet Oncol. 2018, 19, e295–e304.
- Drago, L.; Toscano, M.; Rodighiero, V.; De Vecchi, E.; Mogna, G. Cultivable and Pyrosequenced Fecal Microflora in Centenarians and Young Subjects. J. Clin. Gastroenterol. 2012, 46, S81–S84.
- Jackson, M.A.; Jeffery, I.B.; Beaumont, M.; Bell, J.T.; Clark, A.G.; Ley, R.E.; O’Toole, P.W.; Spector, T.D.; Steves, C.J. Signatures of early frailty in the gut microbiota. Genome Med. 2016, 8, 1–11.
- Jeffery, I.; Lynch, D.B.; O’Toole, P.W. Composition and temporal stability of the gut microbiota in older persons. ISME J. 2015, 10, 170–182.
- Krautkramer, K.A.; Fan, J.; Bäckhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Genet. 2020, 19, 77–94.
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286.
- Keenan, M.J.; Marco, M.L.; Ingram, D.K.; Martin, R.J. Improving healthspan via changes in gut microbiota and fermentation. AGE 2015, 37, 1–10.
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979.
- Vaiserman, A.; Romanenko, M.; Piven, L.; Moseiko, V.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Koliada, A. Differences in the gut Firmicutes to Bacteroidetes ratio across age groups in healthy Ukrainian population. BMC Microbiol. 2020, 20, 1–8.
- Fernandes, J.J.D.R.; Su, W.; Rahat-Rozenbloom, S.; Wolever, T.M.S.; Comelli, E. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr. Diabetes 2014, 4, e121.
- Saccon, T.D.; Nagpal, R.; Yadav, H.; Cavalcante, M.B.; Nunes, A.D.D.C.; Schneider, A.; Gesing, A.; Hughes, B.; Yousefzadeh, M.; Tchkonia, T.; et al. Senolytic Combination of Dasatinib and Quercetin Alleviates Intestinal Senescence and Inflammation and Modulates the Gut Microbiome in Aged Mice. J. Gerontol. Ser. A 2021, 76, 1895–1905.
- Martínez, G.P.; Bäuerl, C.; Collado, M. Understanding gut microbiota in elderly’s health will enable intervention through probiotics. Benef. Microbes 2014, 5, 235–246.
- Lowry, C.A.; Smith, D.G.; Siebler, P.H.; Schmidt, D.; Stamper, C.E.; Hassell, J.E.; Yamashita, P.S.; Fox, J.H.; Reber, S.O.; Brenner, L.A.; et al. The Microbiota, Immunoregulation, and Mental Health: Implications for Public Health. Curr. Environ. Heal. Rep. 2016, 3, 270–286.
- Szántó, M.; Dózsa, A.; Antal, D.; Szabó, K.; Kemény, L.; Bai, P. Targeting the gut-skin axis—Probiotics as new tools for skin disorder management? Exp. Dermatol. 2019, 28, 1210–1218.
- Dinan, T.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2016, 595, 489–503.
- Saarialho-Kere, U. The Gut-Skin Axis. J. Pediatr. Gastroenterol. Nutr. 2004, 39, S734–S735.
- Greuter, T.; Navarini, A.; Vavricka, S.R. Skin Manifestations of Inflammatory Bowel Disease. Clin. Rev. Allergy Immunol. 2017, 53, 413–427.
- Miyazaki, K.; Masuoka, N.; Kano, M.; Iizuka, R. Bifidobacterium fermented milk and galacto-oligosaccharides lead to improved skin health by decreasing phenols production by gut microbiota. Benef. Microbes 2014, 5, 121–128.
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The Gut Microbiome as a Major Regulator of the Gut-Skin Axis. Front. Microbiol. 2018, 9, 1459.
- Sharma, D.; Kober, M.-M.; Bowe, W.P. Anti-Aging Effects of Probiotics. J. Drugs Dermatol. 2016, 15, 9–12.
- Lee, K.; Kim, H.; Kim, S.; Park, S.-D.; Shim, J.-J.; Lee, J.-L. Exopolysaccharide from Lactobacillus plantarum HY7714 Protects against Skin Aging through Skin–Gut Axis Communication. Molecules 2021, 26, 1651.
- Ra, J.; Lee, D.E.; Kim, S.H.; Jeong, J.-W.; Ku, H.K.; Kim, T.-Y.; Choi, I.-D.; Jeung, W.; Sim, J.-H.; Ahn, Y.-T. Effect of Oral Administration of Lactobacillus plantarum HY7714 on Epidermal Hydration in Ultraviolet B-Irradiated Hairless Mice. J. Microbiol. Biotechnol. 2014, 24, 1736–1743.
- Satoh, T.; Murata, M.; Iwabuchi, N.; Odamaki, T.; Wakabayashi, H.; Yamauchi, K.; Abe, F.; Xiao, J. Effect of Bifidobacterium breve B-3 on skin photoaging induced by chronic UV irradiation in mice. Benef. Microbes 2015, 6, 497–504.
- Lee, D.E.; Huh, C.-S.; Ra, J.; Choi, I.-D.; Jeong, J.-W.; Kim, S.-H.; Ryu, J.H.; Seo, Y.K.; Koh, J.S.; Lee, J.-H.; et al. Clinical Evidence of Effects of Lactobacillus plantarum HY7714 on Skin Aging: A Randomized, Double Blind, Placebo-Controlled Study. J. Microbiol. Biotechnol. 2015, 25, 2160–2168.
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868.
- Philips, N.; Auler, S.; Hugo, R.; Gonzalez, S. Beneficial Regulation of Matrix Metalloproteinases for Skin Health. Enzym. Res. 2011, 2011, 427285.
- Baldwin, H.E.; Bhatia, N.D.; Friedman, A.; Eng, R.M.; Seite, S. The Role of Cutaneous Microbiota Harmony in Maintaining a Functional Skin Barrier. J. Drugs Dermatol. 2017, 16, 12–18.
- Nakatsuji, T.; Chen, T.H.; Narala, S.; Chun, K.A.; Two, A.M.; Yun, T.; Shafiq, F.; Kotol, P.F.; Bouslimani, A.; Melnik, A.V.; et al. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci. Transl. Med. 2017, 9, eaah4680.
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin barrier immunity and ageing. Immunology 2019, 160, 116–125.
- Dimitriu, P.A.; Iker, B.; Malik, K.; Leung, H.; Mohn, W.W.; Hillebrand, G.G. New Insights into the Intrinsic and Extrinsic Factors That Shape the Human Skin Microbiome. Mbio 2019, 10, e00839-19.
- Shibagaki, N.; Suda, W.; Clavaud, C.; Bastien, P.; Takayasu, L.; Iioka, E.; Kurokawa, R.; Yamashita, N.; Hattori, Y.; Shindo, C.; et al. Aging-related changes in the diversity of women’s skin microbiomes associated with oral bacteria. Sci. Rep. 2017, 7, 10567.
- Jugé, R.; Rouaud-Tinguely, P.; Breugnot, J.; Servaes, K.; Grimaldi, C.; Roth, M.-P.; Coppin, H.; Closs, B. Shift in skin microbiota of Western European women across aging. J. Appl. Microbiol. 2018, 125, 907–916.
- Somboonna, N.; Wilantho, A.; Srisuttiyakorn, C.; Assawamakin, A.; Tongsima, S. Bacterial communities on facial skin of teenage and elderly Thai females. Arch. Microbiol. 2017, 199, 1035–1042.
- Howard, B.; Bascom, C.C.; Hu, P.; Binder, R.L.; Fadayel, G.; Huggins, T.G.; Jarrold, B.B.; Osborne, R.; Rocchetta, H.L.; Swift, D.; et al. Aging-Associated Changes in the Adult Human Skin Microbiome and the Host Factors that Affect Skin Microbiome Composition. J. Investig. Dermatol. 2021, 142, 1934–1946.
- Mukherjee, S.; Mitra, R.; Maitra, A.; Gupta, S.; Kumaran, S.; Chakrabortty, A.; Majumder, P.P. Sebum and Hydration Levels in Specific Regions of Human Face Significantly Predict the Nature and Diversity of Facial Skin Microbiome. Sci. Rep. 2016, 6, 36062.
This entry is offline, you can click here to edit this entry!
