Metformin is an oral biguanide which has been first-line treatment for type 2 diabetes for several years, however, research has showed that patients undergoing treatment with metformin have a decreased risk for cancer. Interestingly, the compund exhibits a considerable number of antitumor effects which could potentially improve lung cancer treatment. Nonetheless, data regarding the use of metformin as part of the therapeutic scheme for patients with lung cancer has been inconsistent to date. One of the points that the current literature fails to address is the differential effects of metformin in lean vs. obese subjects, which is well established in its use for diabetes, as well as its newly described mechanism of action which depends on redox status of the tumor cell.
- metformin
- body mass index
- fatty acid oxidation
1. Metformin
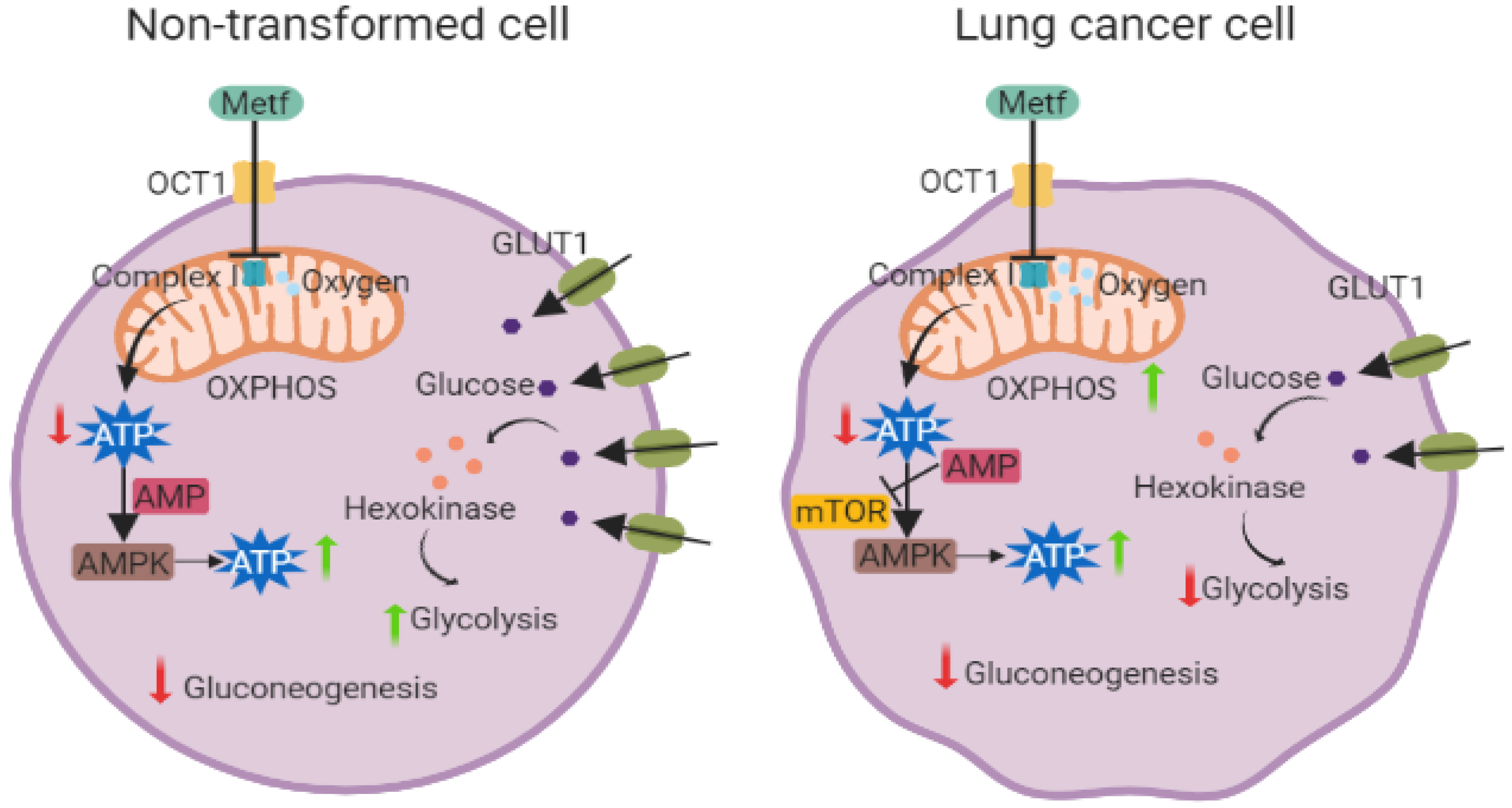 Figure 1. Metformin effects on non-transformed cells versus lung cancer cells. Metformin treatment has different effects on LC cells than non-transformed cells. In both cell types, metformin exerts its effect through a dysfunction of complex I of the electron transport chain, however, in non-transformed cells, metformin promotes glucose incorporation through increased expression of GLUT1 (green membrane proteins), hexokinases (orange circles), and decreasing the gluconeogenesis process. The ATP generation process is also increased through activation of AMPK and with a sustained OXPHOS. In LC cells, metformin stimulates energy generation through over-activation of OXPHOS processes associated with an increasing of AMPK pathway activity, mTOR inhibition, and a decrease in all glycolytic proteins and processes that are associated with this metabolic pathway. (Created with BioRender.com).
Figure 1. Metformin effects on non-transformed cells versus lung cancer cells. Metformin treatment has different effects on LC cells than non-transformed cells. In both cell types, metformin exerts its effect through a dysfunction of complex I of the electron transport chain, however, in non-transformed cells, metformin promotes glucose incorporation through increased expression of GLUT1 (green membrane proteins), hexokinases (orange circles), and decreasing the gluconeogenesis process. The ATP generation process is also increased through activation of AMPK and with a sustained OXPHOS. In LC cells, metformin stimulates energy generation through over-activation of OXPHOS processes associated with an increasing of AMPK pathway activity, mTOR inhibition, and a decrease in all glycolytic proteins and processes that are associated with this metabolic pathway. (Created with BioRender.com).2. Molecular Effects of Metformin in Lung Cancer
2.1. AMPK-Dependent Mechanisms
| Model | AMPK Modification | Treatment | Cell Metabolic Effects | Other Cell Effects | Reference |
|---|---|---|---|---|---|
| A549 and H460 cell lines | Activation | Metformin 20, 40, 80 mM | Not reported | Lung cancer cell cytotoxicity through AMPK/PKA/GSK-3β axis and mediated surviving degradation | [48] |
| A549 and H460 cell lines | Activation | Metformin 1mM for A549 and 2 mM for H460. Cisplatin 1 µM | Not reported | Increased apoptosis in H460 cell line in an AMPK-dependent manner | [49] |
| Lung cancer cells KLN205 | Increased expression and activation | Metformin 5 mM in combination with 5-ALA-PDT 5 J/cm2 | Not reported | Increased cytotoxicity, condensation of nuclear chromatin, and autophagosome formation | [50] |
| A549 cell line | Increased expression and activation | Metformin 0–10 mM in a combination with 2-deoxyglucose 0–2 mM | Lipid peroxidation, decreased glutathione level, super oxide dismutase and catalase activities | Enhanced cytotoxicity, DNA adduct formation, and ROS levels. Increased apoptosis and caspase-3 activity | [51] |
| H460 and H1299 cell lines | AMPK phosphorylation | Metformin 0–10 mM | Not reported | Cell cycle arrest, increased apoptosis, and decreased mTOR activity | [52] |
| A549, H460, H358 and H838 cell lines | Activation | Metformin in combination with sorafenib | Decrease in ROS production, and intracellular glutathione depletion | Antiproliferative effect associated with mTOR pathway inhibition | [53] |
2.2. AMPK-Independent Mechanisms
3. Metformin in Lung Cancer Therapy
3.1. Metformin as an Adjuvant in Lung Cancer Therapy
3.1.1. Metformin plus Platinum-Based Chemotherapy and Radiotherapy
3.1.2. Metformin plus TKIs
3.1.3. Metformin plus Immune Checkpoint Inhibitors
3.1.4 Hypotheses on Metformin, Obesity, and Lung Cancer
The role of metformin in treatment of lung cancer has been difficult to establish thus far. Interestingly, metformin´s salutary effect on this particular neoplasm may play out similarly to its salutary effects in individuals with or without T2D and those with or without obesity. In this regard, early studies pertaining to the use of metformin to stimulate muscular glucose uptake showed metformin increased whole-body insulin-stimulated glucose uptake in patients diagnosed with T2D who presented with obesity [80]. Moreover, this effect could not be exclusively attributed to skeletal muscle uptake, and one possibility involves glucose uptake by adipocytes, which also have insulin-dependent glucose transporters (GLUT4). Clearly, in obese individuals, higher fat mass could in turn aid in glucose clearance. The studies overall indicate that metformin´s improvement of glucose uptake is an indirect consequence of improved glucose control from decreased hepatic glucose production, however, when metformin is used for treatment of non-diabetic individuals with lung cancer many such routes will not play out similarly, since such subjects already have an adequate glycemic control. However, it may be the case that metformin could change nutrient availability, speeding up glucose clearance by peripheral muscle and adipose tissue, thus leaving the tumor cells to achieve energy requirements from other nutrient profiles. Previous studies have shown that in non-diabetic individuals, metformin treatment using a hyperinsulinemic–euglycemic clamp produced increased glucose rate of disappearance and increased glucagon levels [81]. As a result of this profile, non-diabetic patients who are obese would initiate a signaling cascade to mobilize fatty acids stored in adipose tissue cells, through glucagon activation of hormone-sensitive lipase. Many tumors have been reported to readily adapt to lipid-based metabolism, which could predominate in individuals with high availability of such stored nutrients. In a recent study by Rimgel et al. [82], the researchers showed through an elegant single-cell sequencing strategy how tumor cells in obese subjects swiftly change metabolic patterns to improve fat uptake and result in tumor growth. This appears to be at least in part mediated through a decreased expression of PHD3, a prolyl hydroxylase which is relevant in regulating fatty acid oxidation; decreasing PHD3 expression by tumor cells can relieve the suppression of fatty acid transport to the mitochondria, resulting in increased beta-oxidation and ATP production.
However, if a specific tumor lacks this adaptation mechanism the outcome may be deleterious when faced with a higher availability of lipid-based nutrients for which tumor cells compete with other proliferative populations, such as CD8 + lymphocytes. Though PHD3 has not been extensively studied in lung adenocarcinomas, one recent report shows that in samples from surgically resected non-small cell lung cancer, PHD1 and PHD2 mRNA levels are decreased compared with normal tissue, but not PHD3 levels [83]. Whether PHD3 levels in tumors from lean and obese subjects are different remains to be explored, but would be plausible given the transcriptional regulation of this gene by axes such as glucagon–cAMP–PKA signaling in hepatocytes [84]. This could potentially explain the differences seen in the outcomes achieved from metformin treatment of obese vs. lean individuals and could be further explored as a biomarker to predict who could most likely reap benefit from this intervention.
4. Conclusions and Future Directions
Metformin is an oral antidiabetic medication that has been reported to regulate a key sensor of the energetic status of the cells and participate in the inhibition of cellular proliferation by impeding mitosis. Additionally, metformin modulates enzymes that regulate key metabolic pathways and lipid metabolism, leading to the downregulation of cell proliferation, migration, and protein synthesis. Among solid tumors, oxidative phenotype regression through metformin treatment has been broadly associated with a decrease in tumor growth. Metformin may exhibit anticancer properties through the regulation of growth factors such as EGFR and IGF. Metformin has also been shown to suppress epithelial–mesenchymal transition (EMT), impeding the spread of tumor cells. Clinically, the combination of metformin with tyrosine TKIs has been shown to improve the therapeutic response rate as well as OS in specific subgroups of NSCLC patients, particularly those with a high BMI. In addition, treatment with metformin can overcome therapeutic chemo- and TKI-resistance. However, the addition of metformin to conventional cancer therapy is still controversial, indicating that the potential benefit of this combination should be explored according to the novel data emerging regarding biomarkers and patient subgroups.
In this sense, it is necessary to find a potential biomarker to select patients that could benefit from metformin treatment. Loss of expression of LKB1 and AMPK have emerged as a response biomarker in NSCLC patients. Patients with loss of LKB1 with KRAS or EGFR mutations denote an aggressive tumor phenotype, that might be due to the loss of LKB1 as a metabolic sensor; these patients might benefit from biguanide treatment. In addition, AMPK is a potential metformin target, this receptor is lost in NSCLC, and recent reports show that activation of AMPK by metformin decreases PDL-1 levels, which in turn increases cytotoxic T cell activity against cancer cells. Lastly, the role of PHD3 which has emerged as an important regulator of the metabolic switch to lipids by specific tumors must be explored in this clinical context.
The question of which cellular processes and therapies can be potentiated by metformin still remains; therefore, it is necessary to determine the best combination strategies including metformin, the optimal dose to avoid adverse events, and lastly the potential biomarkers to implement it in the context of precision oncology.
This entry is adapted from the peer-reviewed paper 10.3390/ph15070786
References
- American Diabetes Association. Standards of Medical Care in Diabetes—2021. Diabetes Care. 1 January 2021, 44 (Supplement 1). Available online: https://www.diabetes.org/newsroom/press-releases/2020/ADA-releases-2021-standards-of-medical-care-in-diabetes (accessed on 17 May 2022).
- LaMoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2021, 42, 77–96.
- Bharath, L.P.; Agrawal, M.; McCambridge, G.; Nicholas, D.A.; Hasturk, H.; Liu, J.; Jiang, K.; Liu, R.; Guo, Z.; Deeney, J.; et al. Metformin Enhances Autophagy and Normalizes Mitochondrial Function to Alleviate Aging-Associated Inflammation. Cell Metab. 2020, 32, 44–55.e6.
- Pryor, R.; Cabreiro, F. Repurposing metformin: An old drug with new tricks in its binding pockets. Biochem. J. 2015, 471, 307–322.
- Gunton, J.E.; Delhanty, P.J.; Takahashi, S.; Baxter, R.C. Metformin rapidly increases insulin receptor activation in human liver and signals preferentially through insulin-receptor substrate-2. J. Clin. Endocrinol. Metab. 2003, 88, 1323–1332.
- Pernicova, I.; Korbonits, M. Metformin–mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156.
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014, 20, 953–966.
- Graham, G.G.; Punt, J.; Arora, M.; Day, R.O.; Doogue, M.P.; Duong, J.K.; Furlong, T.J.; Greenfield, J.R.; Greenup, L.C.; Kirkpatrick, C.M.; et al. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 2011, 50, 81–98.
- McCreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the gastrointestinal tract. Diabetologia 2016, 59, 426–435.
- Haberkorn, B.; Fromm, M.F.; Konig, J. Transport of Drugs and Endogenous Compounds Mediated by Human OCT1: Studies in Single- and Double-Transfected Cell Models. Front. Pharmacol. 2021, 12, 662535.
- Khodadadi, M.; Jafari-Gharabaghlou, D.; Zarghami, N. An update on mode of action of metformin in modulation of meta-inflammation and inflammaging. Pharmacol. Rep. 2022, 74, 310–322.
- Schlienger, J.L.; Frick, A.; Marbach, J.; Freund, H.; Imler, M. Effects of biguanides on the intermediate metabolism of glucose in normal and portal-strictured rats. Diabete Metab. 1979, 5, 5–9.
- Cusi, K.; Consoli, A.; DeFronzo, R.A. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1996, 81, 4059–4067.
- Luengo, A.; Sullivan, L.B.; Heiden, M.G. Understanding the complex-I-ty of metformin action: Limiting mitochondrial respiration to improve cancer therapy. BMC Biol. 2014, 12, 82.
- Andrzejewski, S.; Siegel, P.M.; St-Pierre, J. Metabolic Profiles Associated with Metformin Efficacy in Cancer. Front. Endocrinol. 2018, 9, 372.
- Andrzejewski, S.; Gravel, S.P.; Pollak, M.; St-Pierre, J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab. 2014, 2, 12.
- Chomanicova, N.; Gazova, A.; Adamickova, A.; Valaskova, S.; Kyselovic, J. The role of AMPK/mTOR signaling pathway in anticancer activity of metformin. Physiol. Res. 2021, 70, 501–508.
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK activators: Mechanisms of action and physiological activities. Exp. Mol. Med. 2016, 48, e224.
- Carling, D. AMPK signalling in health and disease. Curr. Opin. Cell Biol. 2017, 45, 31–37.
- Zhang, Y.L.; Guo, H.; Zhang, C.S.; Lin, S.Y.; Yin, Z.; Peng, Y.; Luo, H.; Shi, Y.; Lian, G.; Zhang, C.; et al. AMP as a low-energy charge signal autonomously initiates assembly of AXIN-AMPK-LKB1 complex for AMPK activation. Cell Metab. 2013, 18, 546–555.
- Sahra, I.B.; Regazzetti, C.; Robert, G.; Laurent, K.; le Marchand-Brustel, Y.; Auberger, P.; Tanti, J.F.; Giorgetti-Peraldi, S.; Bost, F. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res. 2011, 71, 4366–4372.
- Foretz, M.; Hebrard, S.; Leclerc, J.; Zarrinpashneh, E.; Soty, M.; Mithieux, G.; Sakamoto, K.; Andreelli, F.; Viollet, B. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Investig. 2010, 120, 2355–2369.
- Huang, X.; Wullschleger, S.; Shpiro, N.; McGuire, V.A.; Sakamoto, K.; Woods, Y.L.; McBurnie, W.; Fleming, S.; Alessi, D.R. Important role of the LKB1-AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem. J. 2008, 412, 211–221.
- Baas, A.F.; Boudeau, J.; Sapkota, G.P.; Smit, L.; Medema, R.; Morrice, N.A.; Alessi, D.R.; Clevers, H.C. Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD. EMBO J. 2003, 22, 3062–3072.
- Vernieri, C.; Signorelli, D.; Galli, G.; Ganzinelli, M.; Moro, M.; Fabbri, A.; Tamborini, E.; Marabese, M.; Caiola, E.; Broggini, M.; et al. Exploiting FAsting-mimicking Diet and MEtformin to Improve the Efficacy of Platinum-pemetrexed Chemotherapy in Advanced LKB1-inactivated Lung Adenocarcinoma: The FAME Trial. Clin. Lung Cancer 2019, 20, e413–e417.
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174.
- Madiraju, A.K.; Qiu, Y.; Perry, R.J.; Rahimi, Y.; Zhang, X.M.; Zhang, D.; Camporez, J.G.; Cline, G.W.; Butrico, G.M.; Kemp, B.E.; et al. Metformin inhibits gluconeogenesis via a redox-dependent mechanism in vivo. Nat. Med. 2018, 24, 1384–1394.
- Duncan, R.E.; Ahmadian, M.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 2007, 27, 79–101.
- LMcCreight, J.; Mari, A.; Coppin, L.; Jackson, N.; Umpleby, A.M.; Pearson, E.R. Metformin increases fasting glucose clearance and endogenous glucose production in non-diabetic individuals. Diabetologia 2020, 63, 444–447.
- Imai, H.; Kaira, K.; Mori, K.; Ono, A.; Akamatsu, H.; Matsumoto, S.; Taira, T.; Kenmotsu, H.; Harada, H.; Naito, T.; et al. Prognostic significance of diabetes mellitus in locally advanced non-small cell lung cancer. BMC Cancer 2015, 15, 989.
- Nakazawa, K.; Kurishima, K.; Tamura, T.; Ishikawa, H.; Satoh, H.; Hizawa, N. Survival difference in NSCLC and SCLC patients with diabetes mellitus according to the first-line therapy. Med. Oncol. 2013, 30, 367.
- Satoh, H.; Ishikawa, H.; Kurishima, K.; Ohtsuka, M.; Sekizawa, K. Diabetes is not associated with longer survival in patients with lung cancer. Arch. Intern. Med. 2001, 161, 485.
- Landman, G.W.; Kleefstra, N.; van Hateren, K.J.; Groenier, K.H.; Gans, R.O.; Bilo, H.J. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care 2010, 33, 322–326.
- Evans, J.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005, 330, 1304–1305.
- Arrieta, O.; Varela-Santoyo, E.; Soto-Perez-de-Celis, E.; Sanchez-Reyes, R.; de la Torre-Vallejo, M.; Muniz-Hernandez, S.; Cardona, A.F. Metformin use and its effect on survival in diabetic patients with advanced non-small cell lung cancer. BMC Cancer 2016, 16, 633.
- Kong, F.; Gao, F.; Liu, H.; Chen, L.; Zheng, R.; Yu, J.; Li, X.; Liu, G.; Jia, Y. Metformin use improves the survival of diabetic combined small-cell lung cancer patients. Tumour Biol. 2015, 36, 8101–8106.
- Tomic, T.; Botton, T.; Cerezo, M.; Robert, G.; Luciano, F.; Puissant, A.; Gounon, P.; Allegra, M.; Bertolotto, C.; Bereder, J.M.; et al. Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell Death Dis. 2011, 2, e199.
- Hemminki, A. The molecular basis and clinical aspects of Peutz-Jeghers syndrome. Cell Mol. Life Sci. 1999, 55, 735–750.
- Sanchez-Cespedes, M.; Parrella, P.; Esteller, M.; Nomoto, S.; Trink, B.; Engles, J.M.; Westra, W.H.; Herman, J.G.; Sidransky, D. Inactivation of LKB1/STK11 is a common event in adenocarcinomas of the lung. Cancer Res. 2002, 62, 3659–3662.
- Katajisto, P.; Vallenius, T.; Vaahtomeri, K.; Ekman, N.; Udd, L.; Tiainen, M.; Makela, T.P. The LKB1 tumor suppressor kinase in human disease. Biochim. Biophys. Acta 2007, 1775, 63–75.
- Ding, L.; Getz, G.; Wheeler, D.A.; Mardis, E.R.; McLellan, M.D.; Cibulskis, K.; Sougnez, C.; Greulich, H.; Muzny, D.M.; Morgan, M.B.; et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature 2008, 455, 1069–1075.
- Jänne, P.A.; Shaw, A.T.; Pereira, J.R.; Jeannin, G.; Vansteenkiste, J.; Barrios, C.; Franke, F.A.; Grinsted, L.; Zazulina, V.; Smith, P.; et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: A randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol. 2013, 14, 38–47.
- Galan-Cobo, A.; Sitthideatphaiboon, P.; Qu, X.; Poteete, A.; Pisegna, M.A.; Tong, P.; Chen, P.H.; Boroughs, L.K.; Rodriguez, M.L.M.; Zhang, W.; et al. LKB1 and KEAP1/NRF2 Pathways Cooperatively Promote Metabolic Reprogramming with Enhanced Glutamine Dependence in KRAS-Mutant Lung Adenocarcinoma. Cancer Res. 2019, 79, 3251–3267.
- Bhatt, V.; Khayati, K.; Hu, Z.S.; Lee, A.; Kamran, W.; Su, X.; Guo, J.Y. Autophagy modulates lipid metabolism to maintain metabolic flexibility for Lkb1-deficient Kras-driven lung tumorigenesis. Genes Dev. 2019, 33, 150–165.
- Canto, C.; Auwerx, J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105.
- Cruz-Bermudez, A.; Vicente-Blanco, R.J.; Laza-Briviesca, R.; Garcia-Grande, A.; Laine-Menendez, S.; Gutierrez, L.; Calvo, V.; Romero, A.; Martin-Acosta, P.; Garcia, J.M.; et al. PGC-1alpha levels correlate with survival in patients with stage III NSCLC and may define a new biomarker to metabolism-targeted therapy. Sci. Rep. 2017, 7, 16661.
- Aatsinki, S.M.; Buler, M.; Salomaki, H.; Koulu, M.; Pavek, P.; Hakkola, J. Metformin induces PGC-1alpha expression and selectively affects hepatic PGC-1alpha functions. Br. J. Pharmacol. 2014, 171, 2351–2363.
- Luo, Z.; Chen, W.; Wu, W.; Luo, W.; Zhu, T.; Guo, G.; Zhang, L.; Wang, C.; Li, M.; Shi, S. Metformin promotes survivin degradation through AMPK/PKA/GSK-3β-axis in non-small cell lung cancer. J. Cell. Biochem. 2019, 120, 11890–11899.
- Riaz, M.A.; Sak, A.; Erol, Y.B.; Groneberg, M.; Thomale, J.; Stuschke, M. Metformin enhances the radiosensitizing effect of cisplatin in non-small cell lung cancer cell lines with different cisplatin sensitivities. Sci. Rep. 2019, 9, 1282.
- Osaki, T.; Yokoe, I.; Takahashi, K.; Inoue, K.; Ishizuka, M.; Tanaka, T.; Azuma, K.; Murahata, Y.; Tsuka, T.; Itoh, N.; et al. Metformin enhances the cytotoxicity of 5-aminolevulinic acid-mediated photodynamic therapy in vitro. Oncol. Lett. 2017, 14, 1049–1053.
- Hou, X.B.; Li, T.H.; Ren, Z.P.; Liu, Y. Combination of 2-deoxy d-glucose and metformin for synergistic inhibition of non-small cell lung cancer: A reactive oxygen species and P-p38 mediated mechanism. Biomed. Pharmacother. 2016, 84, 1575–1584.
- Guo, Q.; Liu, Z.; Jiang, L.; Liu, M.; Ma, J.; Yang, C.; Han, L.; Nan, K.; Liang, X. Metformin inhibits growth of human non-small cell lung cancer cells via liver kinase B-1-independent activation of adenosine monophosphate-activated protein kinase. Mol. Med. Rep. 2016, 13, 2590–2596.
- Groenendijk, F.H.; Mellema, W.W.; van der Burg, E.; Schut, E.; Hauptmann, M.; Horlings, H.M.; Willems, S.M.; van den Heuvel, M.M.; Jonkers, J.; Smit, E.F.; et al. Sorafenib synergizes with metformin in NSCLC through AMPK pathway activation. Int. J. Cancer Mar. 2015, 136, 1434–1444.
- Janjetovic, K.; Harhaji-Trajkovic, L.; Misirkic-Marjanovic, M.; Vucicevic, L.; Stevanovic, D.; Zogovic, N.; Sumarac-Dumanovic, M.; Micic, D.; Trajkovic, V. In vitro and in vivo anti-melanoma action of metformin. Eur. J. Pharmacol. 2011, 668, 373–382.
- Yousef, M.; Tsiani, E. Metformin in Lung Cancer: Review of in Vitro and in Vivo Animal Studies. Cancers 2017, 9, 45.
- Chae, Y.K.; Arya, A.; Malecek, M.K.; Shin, D.S.; Carneiro, B.; Chandra, S.; Kaplan, J.; Kalyan, A.; Altman, J.K.; Platanias, L.; et al. Repurposing metformin for cancer treatment: Current clinical studies. Oncotarget 2016, 7, 40767–40780.
- Pan, Y.H.; Jiao, L.; Lin, C.Y.; Lu, C.H.; Li, L.; Chen, H.Y.; Wang, Y.B.; He, Y. Combined treatment with metformin and gefitinib overcomes primary resistance to EGFR-TKIs with EGFR mutation via targeting IGF-1R signaling pathway. Biologics 2018, 12, 75–86.
- Tian, Y.; Tang, B.; Wang, C.; Sun, D.; Zhang, R.; Luo, N.; Han, Z.; Liang, R.; Gao, Z.; Wang, L. Metformin mediates resensitivity to 5-fluorouracil in hepatocellular carcinoma via the suppression of YAP. Oncotarget 2016, 7, 46230–46241.
- Honjo, S.; Ajani, J.A.; Scott, A.W.; Chen, Q.; Skinner, H.D.; Stroehlein, J.; Johnson, R.L.; Song, S. Metformin sensitizes chemotherapy by targeting cancer stem cells and the mTOR pathway in esophageal cancer. Int. J. Oncol. 2014, 45, 567–574.
- Arrieta, O.; Zatarain-Barron, Z.L.; Cardona, A.F.; Corrales, L.; Martin, C.; Cuello, M. Uniting Latin America Through Research: How Regional Research Can Strengthen Local Policies, Networking, and Outcomes for Patients with Lung Cancer. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 1–7.
- Lin, C.C.; Yeh, H.H.; Huang, W.L.; Yan, J.J.; Lai, W.W.; Su, W.P.; Chen, H.H.; Su, W.C. Metformin enhances cisplatin cytotoxicity by suppressing signal transducer and activator of transcription-3 activity independently of the liver kinase B1-AMP-activated protein kinase pathway. Am. J. Respir. Cell Mol. Biol. 2013, 49, 241–250.
- Wang, Y.; Lin, B.; Wu, J.; Zhang, H.; Wu, B. Metformin inhibits the proliferation of A549/CDDP cells by activating p38 mitogen-activated protein kinase. Oncol. Lett. 2014, 8, 1269–1274.
- Teixeira, S.F.; Idos, S.G.; Madeira, K.P.; Daltoe, R.D.; Silva, I.V.; Rangel, L.B. Metformin synergistically enhances antiproliferative effects of cisplatin and etoposide in NCI-H460 human lung cancer cells. J. Bras. Pneumol. 2013, 39, 644–649.
- Tan, B.X.; Yao, W.X.; Ge, J.; Peng, X.C.; Du, X.B.; Zhang, R.; Yao, B.; Xie, K.; Li, L.H.; Dong, H.; et al. Prognostic influence of metformin as first-line chemotherapy for advanced nonsmall cell lung cancer in patients with type 2 diabetes. Cancer 2011, 117, 5103–5111.
- Marrone, K.A.; Zhou, X.; Forde, P.M.; Purtell, M.; Brahmer, J.R.; Hann, C.L.; Kelly, R.J.; Coleman, B.; Gabrielson, E.; Rosner, G.L.; et al. A Randomized Phase II Study of Metformin plus Paclitaxel/Carboplatin/Bevacizumab in Patients with Chemotherapy-Naive Advanced or Metastatic Nonsquamous Non-Small Cell Lung Cancer. Oncologist 2018, 23, 859–865.
- Wen-Xiu, X.; Xiao-Wei, Z.; Hai-Ying, D.; Ying-Hui, T.; Si-Si, K.; Xiao-Fang, Z.; Huang, P. Impact of metformin use on survival outcomes in non-small cell lung cancer treated with platinum. Medicine 2018, 97, e13652.
- Koritzinsky, M. Metformin: A Novel Biological Modifier of Tumor Response to Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 454–464.
- Levy, A.; Doyen, J. Metformin for non-small cell lung cancer patients: Opportunities and pitfalls. Crit. Rev. Oncol. Hematol. 2018, 125, 41–47.
- Wink, K.C.; Belderbos, J.S.; Dieleman, E.M.; Rossi, M.; Rasch, C.R.; Damhuis, R.A.; Houben, R.M.; Troost, E.G. Improved progression free survival for patients with diabetes and locally advanced non-small cell lung cancer (NSCLC) using metformin during concurrent chemoradiotherapy. Radiother. Oncol. 2016, 118, 453–459.
- Tsakiridis, T.; Pond, G.R.; Wright, J.; Ellis, P.M.; Ahmed, N.; Abdulkarim, B.; Roa, W.; Robinson, A.; Swaminath, A.; Okawara, G.; et al. Metformin in Combination with Chemoradiotherapy in Locally Advanced Non-Small Cell Lung Cancer: The OCOG-ALMERA Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1333–1341.
- Morgillo, F.; Sasso, F.C.; della Corte, C.M.; Vitagliano, D.; D’Aiuto, E.; Troiani, T.; Martinelli, E.; de Vita, F.; Orditura, M.; de Palma, R.; et al. Synergistic effects of metformin treatment in combination with gefitinib, a selective EGFR tyrosine kinase inhibitor, in LKB1 wild-type NSCLC cell lines. Clin. Cancer Res. 2013, 19, 3508–3519.
- Li, L.; Han, R.; Xiao, H.; Lin, C.; Wang, Y.; Liu, H.; Li, K.; Chen, H.; Sun, F.; Yang, Z.; et al. Metformin sensitizes EGFR-TKI-resistant human lung cancer cells in vitro and in vivo through inhibition of IL-6 signaling and EMT reversal. Clin Cancer Res. 2014, 20, 2714–2726.
- Barrios-Bernal, P.; Hernandez-Pedro, N.; Orozco-Morales, M.; Viedma-Rodriguez, R.; Lucio-Lozada, J.; Avila-Moreno, F.; Cardona, A.F.; Rosell, R.; Arrieta, O. Metformin Enhances TKI-Afatinib Cytotoxic Effect, Causing Downregulation of Glycolysis, Epithelial-Mesenchymal Transition, and EGFR-Signaling Pathway Activation in Lung Cancer Cells. Pharmaceuticals 2022, 15, 381.
- Wang, X.; Chen, K.; Yu, Y.; Xiang, Y.; Kim, J.H.; Gong, W.; Huang, J.; Shi, G.; Li, Q.; Zhou, M.; et al. Metformin sensitizes lung cancer cells to treatment by the tyrosine kinase inhibitor erlotinib. Oncotarget 2017, 8, 109068–109078.
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crino, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135.
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639.
- Eikawa, S.; Nishida, M.; Mizukami, S.; Yamazaki, C.; Nakayama, E.; Udono, H. Immune-mediated antitumor effect by type 2 diabetes drug, metformin. Proc. Natl. Acad. Sci. USA 2015, 112, 1809–1814.
- Ringel, A.E.; Drijvers, J.M.; Baker, G.J.; Catozzi, A.; Garcia-Canaveras, J.C.; Gassaway, B.M.; Miller, B.C.; Juneja, V.R.; Nguyen, T.H.; Joshi, S.; et al. Obesity Shapes Metabolism in the Tumor Microenvironment to Suppress Anti-Tumor Immunity. Cell 2020, 183, 1848–1866.e26.
- Rathmell, J.C. Obesity, Immunity, and Cancer. N. Engl. J. Med. 2021, 384, 1160–1162.
- LaMoia, T.E.; Shulman, G.I. Cellular and Molecular Mechanisms of Metformin Action. Endocr. Rev. 2021, 42, 77–96.
- LMcCreight, J.; Mari, A.; Coppin, L.; Jackson, N.; Umpleby, A.M.; Pearson, E.R. Metformin increases fasting glucose clearance and endogenous glucose production in non-diabetic individuals. Diabetologia 2020, 63, 444–447.
- Chen, H.; Yao, W.; Chu, Q.; Han, R.; Wang, Y.; Sun, J.; Wang, D.; Wang, Y.; Cao, M.; He, Y. Synergistic effects of metformin in combination with EGFR-TKI in the treatment of patients with advanced non-small cell lung cancer and type 2 diabetes. Cancer Lett. 2015, 369, 97–102.
- Koren, A.; Rijavec, M.; Krumpestar, T.; Kern, I.; Sadikov, A.; Cufer, T.; Korosec, P. Gene Expression Levels of the Prolyl Hydroxylase Domain Proteins PHD1 and PHD2 but Not PHD3 Are Decreased in Primary Tumours and Correlate with Poor Prognosis of Patients with Surgically Resected Non-Small-Cell Lung Cancer. Cancers 2021, 13, 2309.
- Yano, H.; Sakai, M.; Matsukawa, T.; Yagi, T.; Naganuma, T.; Mitsushima, M.; Iida, S.; Inaba, Y.; Inoue, H.; Unoki-Kubota, H.; et al. PHD3 regulates glucose metabolism by suppressing stress-induced signalling and optimising gluconeogenesis and insulin signalling in hepatocytes. Sci. Rep. 2018, 8, 14290.
