Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Image-guided percutaneous ablation is defined as the process of percutaneously introducing needlelike applicators to destroy or shrink tumors in a controlled and targeted fashion under image guidance.
- ablation
- tumors
- image-guidance
1. Introduction
Image-guided percutaneous ablation is a well-established minimally invasive practice for primary and metastatic tumors [1,2]. The advent of image-guided percutaneous ablation along with transarterial therapies has allowed interventional radiologists to not only be involved in the diagnosis of cancer but also serve as key players in its treatment and follow-up care [3]. Imaging modalities to guide ablation probes range from ultrasound (US) to magnetic resonance imaging (MRI), and ablation modalities can be divided into several types depending on the form of energy delivery and tissue injury [4]. Given the imaging of cancerous tissues and the damage to the tissue architecture with ablation, this work is applicable to other fields, both within and outside of medicine, that employ deformational analysis [5,6,7].
2. Imaging Modalities for Guiding Percutaneous Ablation Devices
Imaging plays critical roles in the pretreatment diagnosis and monitoring of tumors, peri-treatment placement of the ablation probe, guiding chemical or energy deposition during the procedure, and post-treatment assessment of outcomes [8]. Therefore, the selection of a proper imaging modality is critical for successful ablation. US and computerized tomography (CT) are most commonly employed [9].
2.1. Ultrasound
US is one of the most readily available imaging modalities used for guiding percutaneous ablation devices. It is relatively easy to operate, does not impart ionizing radiation, and provides real-time, multiplanar guidance at a low cost [10,11]. However, the use of US is limited by significant operator dependency, difficulty targeting deep structures in patients with obesity, limited visualization of air containing organs (e.g., intestinal loops), and limited utility for the visualization of tumors such as hepatic or iso-echoic renal tumors [9,10,11,12,13]. Contrast-enhanced ultrasound (CEUS), in which microbubble contrast agents are used as acoustic enhancers, is an alternative modality that enhances procedure guidance and pre- and post-procedural evaluation [14,15]. While CEUS can enhance the visibility of small tumors at a relatively low-cost, it cannot detect all lesions (e.g., deeply seated lesions in the hepatic dome), requires specific operator experience, is limited by the number of contrast injections during each session, and is not widely available [10,16].
2.2. Fluoroscopy
Fluoroscopy is an imaging technique that employs the use of X-ray pulses to capture real-time moving images [17]. Fluoroscopy was the main imaging technique for percutaneous biopsies and drainage procedures before the advent of CT that slowly replaced its use [18]. While useful for providing real-time feedback, fluoroscopy is restricted by its limited ability to navigate out of its plane and the exposure to radiation to both the patient and operator [17].
2.3. Computed Tomography, Cone-Beam CT, and CT Fluoroscopy
CT is the most commonly used imaging modality to guide percutaneous ablation devices as it is widely accessible, has a wide field of view, and is not limited by bowel gas [19]. It is commonly used without contrast; however a limited dose of contrast may be administered to visualize the lesion or identify critical vital structures to avoid non-target injury. The primary disadvantages of CT are ionizing radiation, single plane acquisition (though newer technologies such as IMACTIS CT-Navigation™ System (Hinckley, UK) by BVM Medical, Hinckley, UK are simulating and providing multiplanar views), and limited real-time visualization of iso-dense targets, and requires caution with the use of contrast media in patients with renal insufficiency [9].
Cone-beam computerized tomography (CBCT) is another modality that is well-suited to provide high spatial resolution and 3D image reconstructions. However, CBCT is limited by the relatively longer acquisition time than conventional CT, which can introduce motion artifacts [17]. Fluoroscopy guidance can be paired with CT to provide real-time feedback. It can allow for faster procedural times and improve targeting accuracy by avoiding errors due to patients’ movement and breathing [17]. However, this technique provides a relatively high amount of radiation exposure to both the patient and operator [17]. Other ways to guide percutaneous ablation devices is through the use of lipiodol or radiopaque beads. These are sometimes used in transarterial chemoembolization and can enhance intraprocedural imaging guidance by their ability to be visualized with fluoroscopy or CT [20,21]. Other ways to guide percutaneous ablation devices is via pre-ablation embolization of lesions using lipiodol or radiopaque beads. These are used in transarterial chemoembolization and can enhance intraprocedural imaging guidance by their ability to be visualized by fluoroscopy or CT [20,21].
2.4. MRI
Magnetic resonance imaging (MRI) is less frequently used than US or CT but is useful in that it uses no ionizing radiation, provides a high contrast resolution between soft tissues, displays small tumors with increased sensitivity, allows for imaging in any orientation and on any plane, monitors thermal effects, and can be combined with diffusion-weighted imaging or MRI contrast agents to visualize more difficult lesions [11]. The primary advantage of MRI guidance for tumor ablation, however, lies in its unique thermal sensitivity that allows online monitoring of the progress of ablation. The main disadvantages of MRI use include the lack of MR compatible tools, closed MRI not having real-time guidance, a relatively complicated operation, susceptibility to artifacts, and high cost [11].
3. Physics and Mechanism of Action of Percutaneous Ablation Devices
The tumor ablation techniques are divided into chemical ablation, thermal ablation, irreversible electroporation, or external-energy-delivery-based ablation (Figure 1). Chemical ablation includes nonenergy-based ablation techniques such as intratumoral ethanol and acetic acid injection that cause coagulation necrosis leading to tumor ablation [1,22,23]. Thermal ablation includes modalities that destroy tissue via heat or cold and include radiofrequency ablation (RFA), cryoablation, microwave ablation (MWA), and laser ablation. External-energy-delivery-based ablation includes high-intensity frequency ultrasound (HIFU) and histotripsy (Figure 1). The advantages and disadvantages of the common ablation techniques are summarized in Table 1.
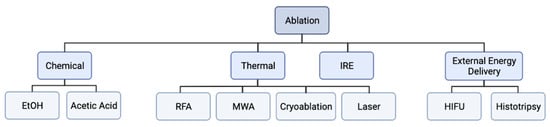
Figure 1. Flowchart of ablation modalities. Ablation modalities can be subdivided into thermal and non-thermal based modalities. Thermal ablation modalities include radiofrequency ablation (RFA), microwave ablation (MWA), cryoablation, and laser ablation. Non-thermal ablation modalities include chemical ablation (ethanol and acetic acid ablation), irreversible electroporation (IRE), and external energy delivery modalities (high intensity focused ultrasound (HIFU) and histotripsy).
Table 1. The advantages and disadvantages of the common ablation techniques.
| Ablation Device | Advantages | Disadvantages |
|---|---|---|
| Ethanol |
|
|
| RFA |
|
|
| Cryoablation |
|
|
| MWA |
|
|
| Laser |
|
|
| IRE |
|
|
| HIFU |
|
|
| Histotripsy |
|
|
3.1. Radiofrequeny Ablation
RFA (Figure 2) works by delivering radiofrequency waves in the 375–500 kHz range to an area surrounding a generator-coupled electrode, causing an oscillating electric field that creates frictional energy by electron collision. This collision generates heat that leads to eventual tumor destruction by coagulation necrosis from temperatures above 60 °C [19,24,25].
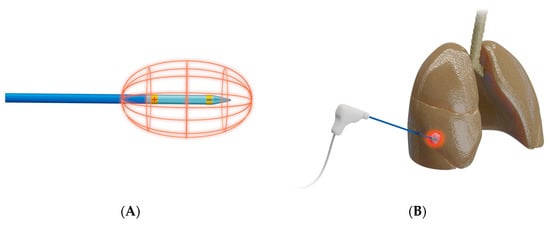
Figure 2. Schematic presentation of radiofrequency ablation. (A) Design of a bipolar RFA system, in which electrical current is delivered through an electrode that functions as both anode and cathode [26]; bi-polar electrodes obviate the need for grounding pads; (B) Representative example of RFA used to target a lesion in right middle lobe of a lung.
3.2. Microwave Ablation
MWA (Figure 3) uses microwave energy in the 300–3000 MHz range from an antenna, creating oscillation of ions in the target [19]. This oscillation creates heat and results in coagulative necrosis. This technique allows for faster ablation times, larger ablation zones, and a reduced heat sink effect compared to RFA [27,28].
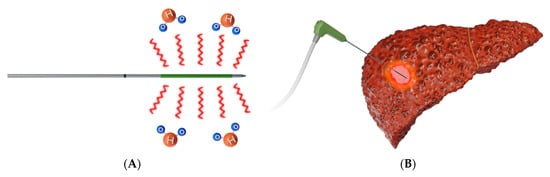
Figure 3. Schematic presentation of microwave ablation. (A) Design of a microwave antenna that generates an oscillating electromagnetic field, leading to the continuous realignment of molecules with an intrinsic dipole moment (mostly water) [26]; (B) Representative example of MWA used to target a liver tumor.
3.3. Cryoablation
Cryoablation (Figure 4) destroys tumors by applying freezing temperatures or alternating freeze–thaw cycles [29]. This technique works by utilizing the Joule–Thomson effect by which certain gases such as nitrogen, nitrous oxide, or argon drop in temperature when going from high pressure to low pressure [24,30]. Once the target region reaches temperature of −40 °C, ice crystals form in the extracellular space that leads to increased tonicity and osmotic damage to cells in the area [24,31,32]. Eventually, intracellular ice forms, rupturing the plasma and organelle membranes, followed by indirect cell death by thrombosis of damaged blood vessels that lead to ischemia and inflammation [24,33].
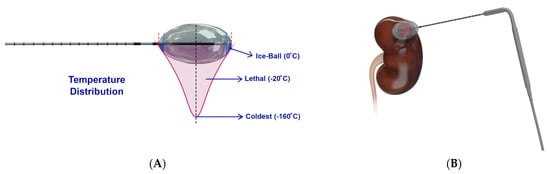
Figure 4. Schematic presentation of cryoablation. (A) Design of a cryoprobe, whereby a cryogen circulates to rapidly cool the cryoprobe to −160 °C, resulting in an ice ball around the cryoprobe of varying temperatures, of which −20 °C is lethal [26]. Figure adapted from Nelson et al. [26]; (B) Representative example of cryoablation used to target a kidney tumor.
3.4. Irreversible Electroporation
IRE (Figure 5) is a nonthermal ablation modality that causes cell death through repeated short-duration high-voltage electrical pulses, leading to ruptured cellular membranes and irreversible cell death [34,35]. IRE differs from the thermal modalities in that it is not affected by the heat sink effect [1].
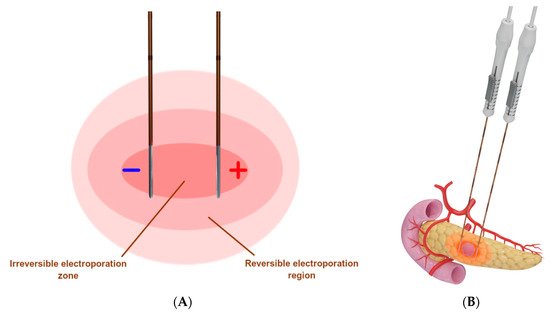
Figure 5. Schematic presentation of irreversible electroporation. (A) Two IRE probes are placed in parallel around the tumor, whereby multiple high-voltage electrical pulses are delivered to produce a uniform zone of ablation [26]; (B) Representative example of IRE used to target a pancreatic tumor.
3.5. Laser Ablation
Laser ablation uses light energy to precisely heat a tumor electromagnetically and cause coagulative necrosis. It is a flexible technique that can be conveniently coupled through optical fibers that are MR compatible, but the small ablation zones require multiple placements [19]. Furthermore, laser ablation can have limited energy penetration as most body tissues absorb and scatter light [36].
3.6. High-Intensity Frequency Ultrasound
HIFU (Figure 6) is a noninvasive thermal ablation technique that causes coagulation necrosis by delivering high-intensity ultrasound waves onto a focal area [37]. The sources of ultrasound are often placed extracorporeally or rectally without the need for the transcutaneous insertion of probes.
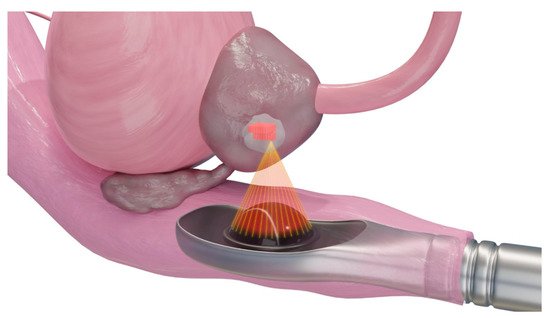
Figure 6. Schematic presentation of high-intensity frequency ultrasound for ablation of a prostate tumor. The extracorporeal transducer generates high-intensity ultrasound to carry and propagate energy onto a focused area deep within tissue media. Temperatures are raised to a level that is sufficient for thermotherapeutic effects, resulting in coagulation necrosis [38,39].
3.7. Histotripsy
Histotripsy (Figure 7) is a noninvasive ablation technique based on ultrasound but is nonthermal. It uses focused ultrasound waves to mechanically destroy tissue through cavitation, causing minimal damage to surrounding tissue [40]. It works by using pulsed sound waves to create “bubble clouds” from gases naturally occurring in the target. These bubbles form and collapse quickly, leading to mechanical force-based destruction of the tissue [41].
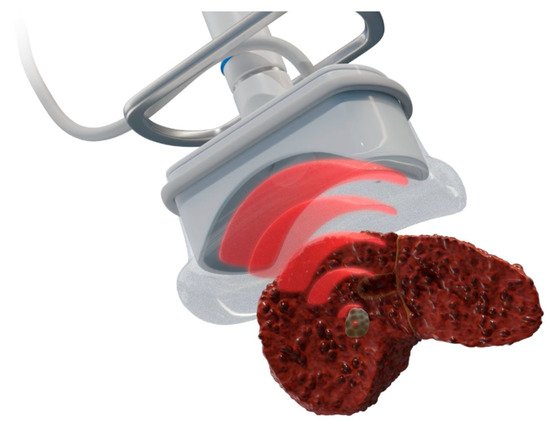
Figure 7. Schematic presentation of histotripsy for liver tumor ablation. Like HIFU, a focused transducer is used to generate and focus ultrasound to the tumor, but it instead causes mechanical damage without thermal coagulation via short, intense acoustic pulses that create dense energetic “bubble clouds” in the tissue media. These bubbles rapidly expand and collapse to mechanically disintegrate tissue [42].
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics12061300
This entry is offline, you can click here to edit this entry!
