Due to high oxygen consumption, the brain is particularly vulnerable to oxidative stress, which is considered an important element in the etiopathogenesis of several mental disorders, including schizophrenia, depression and dependencies. Despite the fact that it is not established yet whether oxidative stress is a cause or a consequence of clinic manifestations, the intake of antioxidant supplements in combination with the psychotropic therapy constitutes a valuable solution in patients’ treatment. When the psychoactive compounds possess themselves antioxidant capacity, this is an added-value for the therapy.
- antipsychotic drugs
- antidepressants
- oxidative stress
- radical scavenging
- serotonin
- fluoxetine
- selenium
- in silico methodologies
- quantum chemistry calculations
- machine learning
1. Introduction
It has been demonstrated that several drugs, or classes of drugs, already in clinical use, are endowed with antioxidant activity. Several classes of approved drugs have been studied through the years for their antioxidant properties [1][2][3]. Proton pump inhibitors [4], antidiabetics [5], drugs acting on the cardiovascular system [6], antiepileptics [7], and anti-inflammatory agents [8] represent some of the investigated classes, which are also paired by antioxidant natural and semi-synthetic compounds with biological activity [9][10][11][12][13][14][15][16]. Furthermore, besides therapeutic agents, it must be stressed that bioactive components from diet have been recognized among the risk factors or, on the other hand, protective agents possibly influencing oxidative stress and pathogenesis of related diseases [17][18]. More specifically, increased peripheral inflammatory markers, elevated production of ROS, reduced activity of the antioxidant systems and decreased efficiency in repairing mechanisms are associated also with mental diseases such as major depressive disorders and schizophrenia, suggesting a direct involvement of oxidative stress in their pathophysiology. While there are numerous studies about oxidative stress and antioxidants, and numerous studies dedicated to psychotropic drugs and their action, much less is known about the antioxidant potential of psychoactive molecules, a topic which is discussed in this work focusing on antipsychotics and antidepressants.
2. Antipsychotic Drugs
Antipsychotic drugs are pharmacological agents that have been introduced over 4 decades ago [19][20]. Currently, treatment options include the use of a single molecule or a combination of substances. These agents are classified as first-generation, or typical, and second-generation, or atypical. Moreover, a third generation of drugs has been more recently introduced [21]. The mechanism of action of typical antipsychotics (haloperidol, 1, Scheme 1) consists in blocking dopamine type 2 receptors. Atypical antipsychotics (clozapine, risperidone, olanzapine, quetiapine and ziprasidone, 2–6, Scheme 1), on the other hand, have lower affinity for dopaminergic receptors but also block serotoninergic 5-HT2A receptors [21]. For more details on the molecular mechanisms underlying the activity of antipsychotic drugs and on the pharmacological aspects, the reader is invited to refer to the recent contributions by Aringhieri and colleagues and by Marder and colleagues [22][23]. Although great improvements in the management of schizophrenia were achieved after the introduction of atypical antipsychotic drugs in the early 1990s, it must also be pointed out that their use is associated with some severe adverse effects. Clozapine can cause agranulocytosis, while the use of olanzapine has been connected with hepatotoxicity [24].
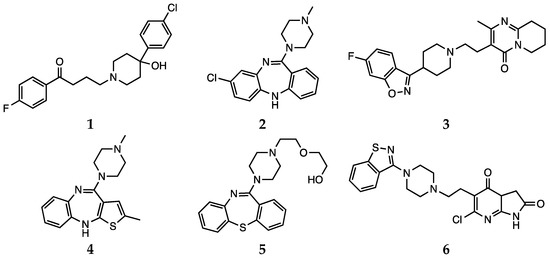
Scheme 1. Haloperidol (1), clozapine (2), risperidone (3), olanzapine (4), quetiapine (5) and ziprasidone (6).
2.1. First Generation (Typical) Antipsychotics
The effects of typical antipsychotics on oxidative stress level is probably the most debated. In fact, evidence suggests that increased lipid peroxidation seems to be associated with the use of these molecules in therapy [25]. Moreover, according to the reports available in the literature, treatment with the typical antipsychotic haloperidol induces a sensible increase in mitochondrial activity in generating toxic reactive species. In particular, the generation of a pyridinium metabolite is thought to be responsible for cytotoxicity, extrapyramidal side effects and cardiac functional disorders [25][26][27]. The antioxidant role of haloperidol was also investigated in a more recent study by Brinholi et al. The compound was not found to be very effective in the in vitro antioxidant tests [20]. Haloperidol was also described to induce lipid peroxidation in schizophrenic patients [28]. The fact that the treatment with such antipsychotics would not lead to unambiguous results is further supported by clinical evidence. Kriisa et al. reported the results of a study conducted considering several indices of oxidative stress in first-episode psychosis patients. The patients were given typical, atypical or mixed medications and oxidative stress markers (total antioxidant capacity, lipid peroxidation and protein oxidation) were measured in blood. First, the authors highlighted the absence of significant differences in such levels between first-episode psychosis patients and the control group. Anyway, the antipsychotic treatment induced two positive effects: a decrease in oxidative status and an amelioration of inflammation. Nevertheless, the authors pointed out that these effects were not observed in long-term chronic schizophrenia patients, who were showing significant high-grade oxidative stress [29].
2.2. Second Generation (Atypical) Antipsychotics
The role of atypical antipsychotics in influencing oxidative stress is also matter of discussion [28]. Some authors reported that changes in antioxidant enzymes concentration and activity, together with other biomarkers of oxidative damage, may be independent of antipsychotic treatment and may otherwise represent the results of the pathophysiological process of the disease in patients [30][31]. In addition to this, and before any other consideration, it must be pointed out that redox behavior and performances of any organic compound depends on several parameters, thus a direct comparison is not always possible. In particular, the results from in vitro and in vivo tests may differ due to a number of reaction conditions. Moreover, as pointed out by Janaszewska and Bartosz, even in the context of a simple and preliminary in vitro test, the antioxidant activity of a given compound may appear different when estimated with different tests, due to peculiar indicators or reaction kinetics [24][32]. It must also be stressed that such antioxidant effect could be direct or indirect (mediated by enzymes or other biochemical pathways). Thus, the antioxidant activity should be tested in several models to better evaluate different possible mechanisms and pathways [20]. Several reports suggested that atypical antipsychotics may improve oxidative status, decreasing damage markers [33][34]. Although the mechanism of action is not completely clear, this effect could be exerted by interfering with antioxidant enzymes or by contrasting O2•− and hydroxyl radical formation [33]. Other reports indicated that atypical antipsychotics act indirectly by increasing the concentration of the serotonergic metabolite 5-hydroxyindol acetic acid, an efficient scavenger of hydroxyl and superoxide radicals that also contrasts lipid peroxidation [35]. Moreover, Sadowska-Bartosz et al. stressed the relevance of the “local antioxidant action” of atypical antipsychotics, due to their higher local concentration in proximity to dopamine and serotonin receptors. This behavior would result in a protective effect against oxidation, nitration and chlorination of receptors themselves, thus allowing correct receptor functioning and signaling [24].
In rodent models, with the exception of olanzapine, treatment with atypical antipsychotics did not induce significant changes in lipid peroxidation levels, which were also detected after 90 days of treatment. Moreover, previous studies demonstrated that olanzapine and other antipsychotics could stimulate the ROS production, glutathione depletion and lipid peroxidation [36][37]. There is also evidence showing that olanzapine may exert antioxidant activity by upregulating SOD [38][39]. Concerning in vivo effects, a general increase in serum total antioxidant status was observed after 2 months of olanzapine treatment, paralleled by a decrease in serum malondialdehyde levels [40]. More recently, Sadowska-Bartosz et al. presented a study focused on the evaluation of the antioxidant properties of atypical antipsychotics in cell-free and cellular systems. Olanzapine and clozapine were identified as the most efficient antioxidants on the basis of a set of tests investigating the effects of such drugs at the molecular level (DHR123 oxidation, ABTS, DPPH, FRAP, fluorescein bleaching), in agreement with previous observations [20]. The authors rationalized these results by discussing the structural features of the two compounds. In fact, the molecules bear similar functional groups, consisting of a nitrogen-containing moiety behaving as Lewis bases capable of donating electrons, thus stabilizing radical species [24]. Clozapine was also observed to be effective in the DPPH radical scavenging test and as a H2O2 inactivator in a previous study [41][42]. These results are further supported by clinical data, such as the observation of the effects of olanzapine and clozapine in patients, where a decrease in radical-induced damage and neurological symptoms was observed after administration [43]. Moreover, olanzapine is thought to improve SOD functioning [34]. On the other hand, it must be considered that a previous study on schizophrenic patients highlighted that clozapine may induce oxidative stress and pro-apoptotic gene expression in neutrophils [44].
2.3. Aripiprazole
In the context of antipsychotic treatments, aripiprazole (7, Scheme 2) shows a different mechanism of action and, consequently, is referred to as a third-generation agent. This drug acts as a partial agonist on D2, D3, and 5-HT1A receptors, while is an antagonist for 5-HT2A receptors. Aripiprazole is the first partial dopamine agonist marketed as an antipsychotic drug, and it is also defined as a dopamine-serotonin system stabilizer [19][37]. It is very effective in treating affective, cognitive and negative symptoms of schizophrenia [45].
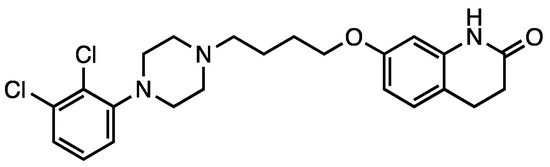
Scheme 2. Aripiprazole (7).
Park et al. reported that aripiprazole, as well as olanzapine and ziprasidone, could provide protection against oxidative stress in a N-methyl-4-phenylpyridinium (MPP+) ion-induced rodent model by modulating ROS levels and SOD activity) and BCL2-associated X protein (Bax) expression [46]. Kato et al. reported that aripiprazole may also contrast microglial O2•− generation by interfering with the cascade of protein kinase C (PKC) activation, intracellular Ca2+ signaling and NADPH oxidase activation [47].
Aripiprazole was also considered by Cai et al. in the study investigating the therapeutic efficacy of antipsychotics in targeting stress-related metabolic pathways mentioned above. This drug, as well as clozapine and risperidone, was found to be effective in regulating creatine levels in prefrontal cortex and hyppocampus [48].
Dietrich-Muszalska et al. compared the in vitro antioxidant effect of aripiprazole with that of other antipsychotic drugs (haloperidol, clozapine, risperidone, olanzapine, quetiapine and ziprasidone) at concentrations corresponding to their clinically effective doses in the plasma of patients. The effect of such treatment was evaluated by measuring TBARS levels, which is an indicator of lipid peroxidation in plasma. According to the findings of these authors, aripiprazole induced insignificant lipid peroxidation in plasma, whereas it showed antioxidant effects on TBARS level in plasma at higher doses [37].
3. Antidepressant Drugs
Also in the case of antidepressants, the results in ameliorating oxidative stress are debated [49]. This may be due to the fact that the class of antidepressants is wide and variegated, comprehending different molecules acting through several mechanisms of action [50]. Depression is a multifaceted disease, neurobiology and molecular events leading to this pathology are still rather unclear.
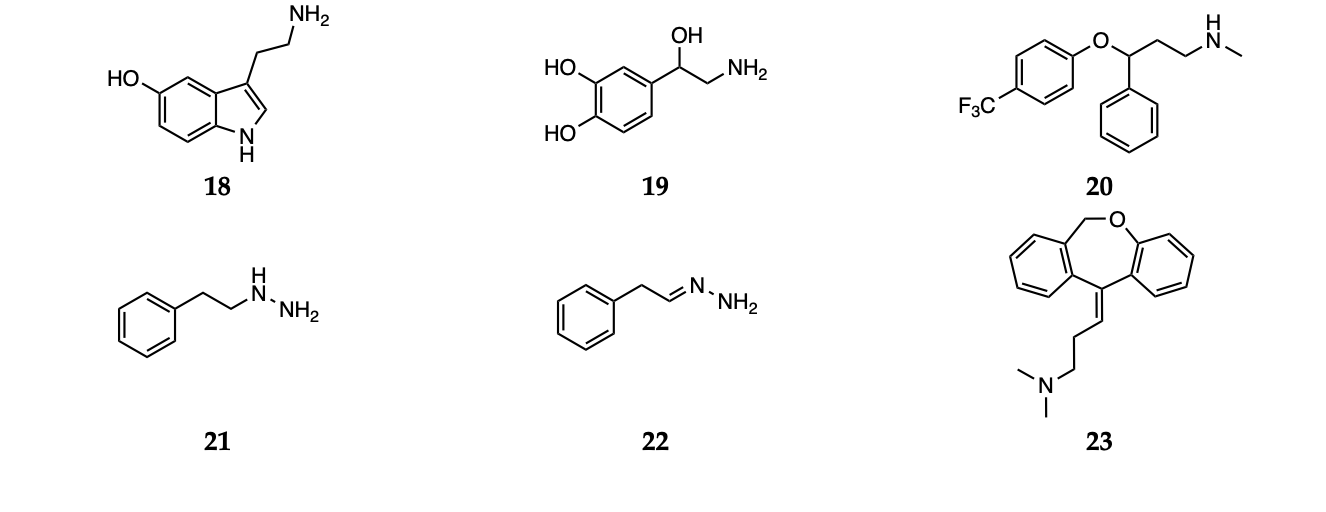
Nevertheless, growing evidences suggest that dietary or commonly administered antioxidants may exert their antidepressant activity by increasing the availability of serotonin (18) and noradrenaline (19, Scheme 3) in the synaptic cleft, thus acting similarly to the conventional antidepressants [51].
Scheme 3 Serotonin (18), noradrenaline (19), fluoxetine (20), phenelzine (21), β-phenylethylidenehydrazine (22) and doxepin (23).
Particularly, serotonin and its balance have been extensively studied from this perspective.
4. Other Agents against Oxidative Stress: Natural and Dietary Compounds
Besides synthetic antipsychotics, other natural and dietary small molecules have been reported to play an antioxidant role and inactivate harmful reactive species in the context of schizophrenia. The administration of PUFAs to rats represents an explicative example, since an increase in SOD activity was observed [52][53]. The potential of vitamin C (water soluble) and vitamin E (lipid soluble) as antioxidant supplement in patients with schizophrenia was also investigated. However,, the use of vitamins C and E does not appear to be a feasible strategy, since the high required dietary intake would most likely result in a pro-oxidant action [54]. Bošković et al. reviewed the contributions reporting studies performed using other supplements, such as N-acetyl cysteine (8), rutin (9), Ginkgo biloba, melatonin (10), hydroxytyrosol (11), caffeic acid phenethyl ester (12), resveratrol (13), quercetin (14) and lycopene (15, Scheme 4) [30]. Various preclinical and clinical studies have shown the positive effects of Ginkgo biloba in enhancing cognitive abilities in impaired individuals and reducing anxiety under pathological conditions [55]. Unluckily, due to data heterogeneity and uncertain mechanisms of action, the correct interpretation of such effects is not trivial.
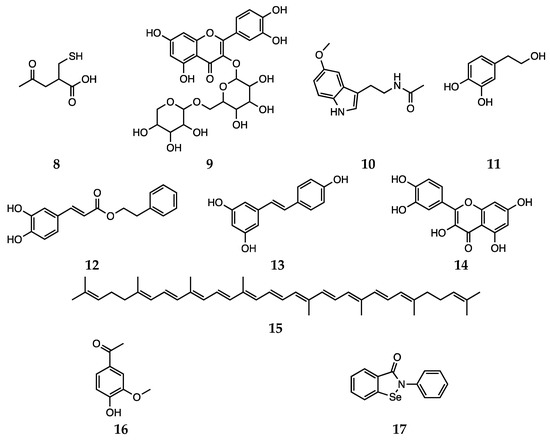
Scheme 4. N-acetyl cysteine (8), rutin (9), melatonin (10), hydroxytyrosol (11), caffeic acid phenethyl ester (12), resveratrol (13), quercetin (14), lycopene (15), apocynin (16) and ebselen (17).
5. In silico approaches
The overview thus far gives a quite good picture of how already existing compounds can have tandem beneficial effect in treating major mental disorders and reducing oxidative stress levels. The employment of computational algorithms developed in the last years to the possible use of antipsychotic or antidepressant drugs as effective antioxidants has also seen a decisive contribution of many researchers [56][57][58][59][60][61][62][63]. However, many of the studies performed so far make use of statistical or classical mechanics based methodologies (like QSAR, molecular docking and molecular dynamics) [64][65][66]. These approaches, while being very useful when dealing with a large number of trial molecules, do not have the ability of elucidating the intrinsic molecular mechanism underlying the efficacy of a particular structure. For this task, one needs resort to methods based on quantum mechanical (QM) calculations, or even better to combine this latter type of accurate calculations with a machine learning rapid and efficient screening.
In a recent study by some of us, [61] the free radical scavenging activity of fluoxetine (20) and serotonin (18, Scheme 3) was investigated using a meta-hybrid functional (M06-2X [67]) in the gas phase and in solvent. The study confirmed the notion that although fluoxetine possesses some radical scavenging capacity on its own, it is less active than serotonin itself. Thus, the effect it exerts as oxidative stress balancer most likely comes from the higher concentration of free serotonin found when the drug is taken. In addition, the employment of DFT computations allowed the authors to analyze the antioxidant activity of each available site for a range of different mechanisms, giving a complete picture of the overall mechanism of antioxidant activity of fluoxetine and serotonin. This is an example of how in silico methodologies allow to investigate the antioxidant capacity of a drug, relating it to specific molecular features and thus providing essential elements for drug design.
This entry is adapted from the peer-reviewed paper 10.3390/antiox9080714
References
- B. Halliwell; Drug antioxidant effect. European Journal of Anaesthesiology 1996, 13, 166, 10.1097/00003643-199603000-00035.
- Fei Fei; Ning Su; Xia Li; Zhou Fei; Neuroprotection mediated by natural products and their chemical derivatives. Neural Regeneration Research 2020, 15, 2008, 10.4103/1673-5374.282240.
- Helmut Sies; Dean P. Jones; Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nature Reviews Molecular Cell Biology 2020, 21, 363-383, 10.1038/s41580-020-0230-3.
- Mohammed N. Abed; Fawaz A. Alassaf; Mahmood H.M. Jasim; Mohanad Alfahad; Mohannad E. Qazzaz; Comparison of Antioxidant Effects of the Proton Pump-Inhibiting Drugs Omeprazole, Esomeprazole, Lansoprazole, Pantoprazole, and Rabeprazole. Pharmacology 2020, null, 1-7, 10.1159/000506232.
- Siu Wai Choi; Cyrus K. Ho; Antioxidant properties of drugs used in Type 2 diabetes management: could they contribute to, confound or conceal effects of antioxidant therapy?. Redox Report 2017, 23, 1-24, 10.1080/13510002.2017.1324381.
- M.E. Neganova; S. G. Klochkov; E. F. Shevtsova; T. N. Bogatyrenko; D. V. Mishchenko; Antioxidant Properties of a Pharmaceutical Substance Hypocard, a Potential Drug for Ischemic Disease. Bulletin of Experimental Biology and Medicine 2018, 166, 46-49, 10.1007/s10517-018-4286-4.
- De Albuquerque Oliveira Aline; Isabel Linhares Maria; Jos Eacute Maia Chaves Filho Adriano; Ricardo Vasconcelos Rios Emiliano; Nayane De Carvalho Lima Camila; Teles Venancio Edith; Gomes De Souza Alana; Alves De Lima Klistenes; Eacute A Floren Ccedil Francisca; Macedo Gaspar Danielle; et al. Antioxidant properties of antiepileptic drugs levetiracetam and clonazepam in mice brain after in vitro-induced oxidative stress. African Journal of Pharmacy and Pharmacology 2016, 10, 278-288, 10.5897/ajpp2015.4358.
- Aderoju Osowole; SYNTHESIS, PHYSICOCHEMICAL AND ANTIOXIDANT PROPERTIES OF SOME METAL(II) COMPLEXES OF MIXED DRUGS, ASPIRIN AND NICOTINAMIDE.. Lettters in Health and Biological Sciences 2016, 2, 1-6, 10.15436/2475-6245.16.010.
- Redaelli, M.; Mucignat-Caretta, C.; Isse, A.A.; Gennaro, A.; Pezzani, R.; Pasquale, R.; Pavan, V.; Crisma, M.; Ribaudo, G.; Zagotto, G. New naphthoquinone derivatives against glioma cells. Eur. J. Med. Chem. 2015, 96, 458–466.
- Wang, F.; Zhang, Y.; Wu, S.; He, Y.; Dai, Z.; Ma, S.; Liu, B. Studies of the structure-antioxidant activity relationships and antioxidant activity mechanism of iridoid valepotriates and their degradation products. PLoS ONE 2017, 12, e0189198.
- Zanforlin, E.; Zagotto, G.; Ribaudo, G. The medicinal chemistry of natural and semisynthetic compounds against Parkinson’s and Huntington’s diseases. ACS Chem. Neurosci. 2017, 8, 2356–2368.
- Pavan, V.; Ribaudo, G.; Zorzan, M.; Redaelli, M.; Pezzani, R.; Mucignat-Caretta, C.; Zagotto, G. Antiproliferative activity of Juglone derivatives on rat glioma. Nat. Prod. Res. 2017, 31, 632–638.
- Pohl, F.; Kong Thoo Lin, P. The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: In vitro, in vivo and clinical trials. Molecules 2018, 23, 3283.
- Povolo, C.; Foschini, A.; Ribaudo, G. Optimization of the extraction of bioactive molecules from Lycium barbarum fruits and evaluation of the antioxidant activity: A combined study. Nat. Prod. Res. 2019, 33, 2694–2698.
- Ribaudo, G.; Ongaro, A.; Zorzan, M.; Pezzani, R.; Redaelli, M.; Zagotto, G.; Memo, M.; Gianoncelli, A. Investigation of the molecular reactivity of bioactive oxiranylmethyloxy anthraquinones. Arch. Pharm. (Weinh.) 2019, 352, 1900030.
- Mastinu, A.; Ribaudo, G.; Ongaro, A.; Bonini, S.A.; Memo, M.; Gianoncelli, A. Critical review on the Chemical Aspects of Cannabidiol (CBD) and harmonization of computational bioactivity data. Curr. Med. Chem. 2020, 27, DOI : 10.2174/0929867327666200210144847.
- Justyna Godos; Walter Currenti; D Angelino; Pedro Mena; Sabrina Castellano; Filippo Caraci; Fabio Galvano; Daniele Del Rio; Raffaele Ferri; Giuseppe Grosso; et al. Diet and Mental Health: Review of the Recent Updates on Molecular Mechanisms. Antioxidants 2020, 9, 346, 10.3390/antiox9040346.
- Ekta Singh; Giles Devasahayam; Neurodegeneration by oxidative stress: a review on prospective use of small molecules for neuroprotection. Molecular Biology Reports 2020, 47, 3133-3140, 10.1007/s11033-020-05354-1.
- Vinay Parikh; Mohammad M Khan; Sahebarao P Mahadik; Differential effects of antipsychotics on expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. Journal of Psychiatric Research 2003, 37, 43-51, 10.1016/S0022-3956(02)00048-1.
- Francis Fregonesi Brinholi; Carine Coneglian De Farias; Kamila Bonifacio; Luciana Higachi; Rúbia Casagrande; Estefânia Gastaldello Moreira; Decio S. Barbosa; Clozapine and olanzapine are better antioxidants than haloperidol, quetiapine, risperidone and ziprasidone in in vitro models. Biomedicine & Pharmacotherapy 2016, 81, 411-415, 10.1016/j.biopha.2016.02.047.
- Paola Dazzan; Kevin Morgan; Ken Orr; Gerard Hutchinson; Xavier Chitnis; John Suckling; Paul Fearon; Philip McGuire; Rosemarie M Mallett; Peter B Jones; et al. Different Effects of Typical and Atypical Antipsychotics on Grey Matter in First Episode Psychosis: the ÆSOP Study. Neuropsychopharmacology 2005, 30, 765-774, 10.1038/sj.npp.1300603.
- Stefano Aringhieri; Marco Carli; Shivakumar Kolachalam; Valeria Verdesca; Enrico Cini; Mario Rossi; Peter J. McCormick; Giovanni U. Corsini; Roberto Maggio; Marco Scarselli; et al. Molecular targets of atypical antipsychotics: From mechanism of action to clinical differences. Pharmacology & Therapeutics 2018, 192, 20-41, 10.1016/j.pharmthera.2018.06.012.
- Stephen R. Marder; Tyrone D. Cannon; Schizophrenia. New England Journal of Medicine 2019, 381, 1753-1761, 10.1056/nejmra1808803.
- Izabela Sadowska-Bartosz; Sabina Galiniak; Grzegorz Bartosz; Mariusz Zuberek; Agnieszka Grzelak; Anna Dietrich-Muszalska; Antioxidant properties of atypical antipsychotic drugs used in the treatment of schizophrenia. Schizophrenia Research 2016, 176, 245-251, 10.1016/j.schres.2016.07.010.
- Stefan Kropp; Veronika Kern; Kirsten Lange; Detlef Degner; Göran Hajak; Johannes Kornhuber; Eckart Rüther; Hinderk M. Emrich; Udo Schneider; Stefan Bleich; et al. Oxidative Stress During Treatment With First- and Second-Generation Antipsychotics. The Journal of Neuropsychiatry and Clinical Neurosciences 2005, 17, 227-231, 10.1176/appi.neuropsych.17.2.227.
- Yutaka Sagara; Induction of reactive oxygen species in neurons by haloperidol.. Journal of Neurochemistry 1998, 71, 1002-1012, 10.1046/j.1471-4159.1998.71031002.x.
- Kazuhiko Iwahashi; K. Anemo; K. Nakamura; I. Fukunishi; K. Igarashi; Analysis of the metabolism of haloperidol and its neurotoxic pyridinium metabolite in patients with drug-induced parkinsonism.. Neuropsychobiology 2001, 44, 126-128, 10.1159/000054931.
- Manuela Padurariu; Alin Ciobica; Lucian Hritcu; Bogdan Stoica; Walther Bild; Cristinel Stefanescu; Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer's disease. Neuroscience Letters 2010, 469, 6-10, 10.1016/j.neulet.2009.11.033.
- Kärt Kriisa; Liina Haring; Eero Vasar; Kati Ekoido; Sven Janno; Veiko Vasar; Kersti Zilmer; Mihkel Zilmer; Antipsychotic Treatment Reduces Indices of Oxidative Stress in First-Episode Psychosis Patients. Oxidative Medicine and Cellular Longevity 2016, 2016, 1-7, 10.1155/2016/9616593.
- Bošković, M.; Vovk, T.; Kores Plesničar, B.; Grabnar, I; Oxidative stress in schizophrenia. Curr. Neuropharmacol 2011, 9, 301-312, .
- Omer Akyol; Hasan Herken; Efkan Uz; Ersin Fadıllıoǧlu; Süheyla Ünal; Sadık Söǧüt; Hüseyin Özyurt; Haluk Asuman Savaş; The indices of endogenous oxidative and antioxidative processes in plasma from schizophrenic patients. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2002, 26, 995-1005, 10.1016/s0278-5846(02)00220-8.
- A. Janaszewska; G. Bartosz; Assay of total antioxidant capacity: comparison of four methods as applied to human blood plasma. Scandinavian Journal of Clinical and Laboratory Investigation 2002, 62, 231-236, 10.1080/003655102317475498.
- Ganesh Dakhale; Suchet Khanzode; Shruti Khanzode; Anand Saoji; Linesh Khobragade; Avinash Turankar; Oxidative damage and schizophrenia: the potential benefit by atypical antipsychotics.. Neuropsychobiology 2004, 49, 205-209, 10.1159/000077368.
- D R Evans; V V Parikh; Mohammad M. Khan; C Coussons; P F Buckley; S P Mahadik; Red blood cell membrane essential fatty acid metabolism in early psychotic patients following antipsychotic drug treatment.. Prostaglandins, Leukotrienes and Essential Fatty Acids 2003, 69, 393-399, 10.1016/j.plefa.2003.08.010.
- Randy D. Blakely; Sherry A. Wages; Joseph B. Justice; James G. Herndon; Darryl B. Neill; Neuroleptics increase striatal catecholamine metabolites but not ascorbic acid in dialyzed perfusate. Brain Research 1984, 308, 1-8, 10.1016/0006-8993(84)90910-7.
- Aziz Eftekhari; Yadollah Azarmi; Alireza Parvizpur; Mohammad Ali Eghbal; Involvement of oxidative stress and mitochondrial/lysosomal cross-talk in olanzapine cytotoxicity in freshly isolated rat hepatocytes. Xenobiotica 2015, 46, 369-378, 10.3109/00498254.2015.1078522.
- Anna Dietrich-Muszalska; Jolanta Kolińska-Łukaszuk; Comparative effects of aripiprazole and selected antipsychotic drugs on lipid peroxidation in plasma. Psychiatry and Clinical Neurosciences 2018, 72, 329-336, 10.1111/pcn.12631.
- Zelan Wei; Ou Bai; J. Steven Richardson; Darrell D. Mousseau; Xin-Min Li; Olanzapine protects PC12 cells from oxidative stress induced by hydrogen peroxide. Journal of Neuroscience Research 2003, 73, 364-368, 10.1002/jnr.10668.
- Xiang Yang Zhang; Dong Feng Zhou; Yu Cun Shen; Pei Yan Zhang; Wu Fang Zhang; Jun Liang; Da Chun Chen; Mei Hong Xiu; Therese A. Kosten; Thomas R. Kosten; et al. Effects of risperidone and haloperidol on superoxide dismutase and nitric oxide in schizophrenia. Neuropharmacology 2012, 62, 1928-1934, 10.1016/j.neuropharm.2011.12.014.
- Basil M. Al-Chalabi; Imad A.J. Thanoon; Faris A. Ahmed; Potential Effect of Olanzapine on Total Antioxidant Status and Lipid Peroxidation in Schizophrenic Patients. Neuropsychobiology 2009, 59, 8-11, 10.1159/000202823.
- A. Dalla Libera; G. Scutari; R. Boscolo; M.P. Rigobello; A. Bindoli; Antioxidant properties of clozapine and related neuroleptics.. Free Radical Research 1998, 29, 151-157, 10.1080/10715769800300171.
- Alzbeta Kracmarova; Miroslav Pohanka; The impact of clozapine on regulation of inflammation in murine macrophage cells.. Neuro endocrinology letters 2014, 35, 175-179, .
- Om Prakash Singh; Indranil Chakraborty; Anindya Dasgupta; Subinay Datta; A comparative study of oxidative stress and interrelationship of important antioxidants in haloperidol and olanzapine treated patients suffering from schizophrenia. Indian Journal of Psychiatry 2008, 50, 171-176, 10.4103/0019-5545.43627.
- Karin Fehsel; Stefan Loeffler; Klaus Krieger; Uwe Henning; Markus Agelink; Victoria Kolb-Bachofen; Ansgar Klimke; Clozapine Induces Oxidative Stress and Proapoptotic Gene Expression in Neutrophils of Schizophrenic Patients. Journal of Clinical Psychopharmacology 2005, 25, 419-426, 10.1097/01.jcp.0000177668.42640.fe.
- Richard B. Mailman; Richard B. Mailman And Vishakantha Murthy; Third Generation Antipsychotic Drugs: Partial Agonism or Receptor Functional Selectivity?. Current Pharmaceutical Design 2010, 16, 488-501, 10.2174/138161210790361461.
- Sung-Woo Park; Chan Hong Lee; Jung Goo Lee; Luck Woo Kim; Bae Sub Shin; Bong Ju Lee; Young Hoon Kim; Protective effects of atypical antipsychotic drugs against MPP+-induced oxidative stress in PC12 cells. Neuroscience Research 2011, 69, 283-290, 10.1016/j.neures.2011.01.004.
- Takahiro A. Kato; Akira Monji; Keiji Yasukawa; Yoshito Mizoguchi; Hideki Horikawa; Yoshihiro Seki; Sadayuki Hashioka; Youn-Hee Han; Mina Kasai; Noriyuki Sonoda; et al. Aripiprazole inhibits superoxide generation from phorbol-myristate-acetate (PMA)-stimulated microglia in vitro: Implication for antioxidative psychotropic actions via microglia. Schizophrenia Research 2011, 129, 172-182, 10.1016/j.schres.2011.03.019.
- H L Cai; Pei Jiang; Q Y Tan; R L Dang; M M Tang; Y Xue; Y Deng; B K Zhang; P F Fang; P Xu; et al. Therapeutic efficacy of atypical antipsychotic drugs by targeting multiple stress-related metabolic pathways.. Translational Psychiatry 2017, 7, e1130-e1130, 10.1038/tp.2017.94.
- Andrea Cipriani; Toshi A Furukawa; Georgia Salanti; Anna Chaimani; Lauren Z Atkinson; Yusuke Ogawa; Stefan Leucht; Henricus G Ruhe; Erick H Turner; Julian P T Higgins; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. The Lancet 2018, 391, 1357-1366, 10.1016/s0140-6736(17)32802-7.
- Alessandra Das Graças Fedoce; Frederico Ferreira; Robert G. Bota; Vicent Bonet-Costa; Patrick Y. Sun; Kay E. Davies; The role of oxidative stress in anxiety disorder: cause or consequence?. Free Radical Research 2018, 52, 737-750, 10.1080/10715762.2018.1475733.
- Jaouad Bouayed; Hassan Rammal; Rachid Soulimani; Oxidative Stress and Anxiety: Relationship and Cellular Pathways. Oxidative Medicine and Cellular Longevity 2009, 2, 63-67, 10.4161/oxim.2.2.7944.
- Sahebarao P. Mahadik; Denise Evans; Harbns Lal; Oxidative stress and role of antioxidant and ω-3 essential fatty acid supplementation in schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2001, 25, 463-493, 10.1016/s0278-5846(00)00181-0.
- Ahmet Songur; Mustafa Sarsilmaz; Sadık Sogut; Birsen Ozyurt; Huseyin Ozyurt; Ismail Zararsiz; Asli Ozdem Turkoglu; Sadik Sogut; Hypothalamic superoxide dismutase, xanthine oxidase, nitric oxide, and malondialdehyde in rats fed with fish ω-3 fatty acids. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2004, 28, 693-698, 10.1016/j.pnpbp.2004.05.006.
- Nikolaus Michael; Hildegard Sourgens; Volker Arolt; Andreas Erfurth; Severe tardive dyskinesia in affective disorders: treatment with vitamin E and C.. Neuropsychobiology 2002, 46, 28-30, 10.1159/000068019.
- Sandeep Kumar Singh; George E Barreto; Gjumrakch Aliev; Valentina Echeverria; Ginkgo biloba as an Alternative Medicine in the Treatment of Anxiety in Dementia and other Psychiatric Disorders. Current Drug Metabolism 2017, 18, 112-119, 10.2174/1389200217666161201112206.
- Zhao, K.; So, H.-C. Drug repositioning for schizophrenia and depression/anxiety disorders: A machine learning approach leveraging expression data. IEEE J. Biomed. Health Inform. 2019, 23, 1304–1315.
- Kumar, A.; Tiwari, A.; Sharma, A. Changing paradigm from one target one ligand towards multi-target directed ligand design for key drug targets of alzheimer disease: An important role of in silico methods in multi-target directed ligands design. Curr. Neuropharmacol. 2018, 16, 726–739.
- Langbein, K.; Hesse, J.; Gussew, A.; Milleit, B.; Lavoie, S.; Amminger, G.P.; Gaser, C.; Wagner, G.; Reichenbach, J.R.; Hipler, U.-C.; et al. Disturbed glutathione antioxidative defense is associated with structural brain changes in neuroleptic-naïve first-episode psychosis patients. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 103–110.
- Casaril, A.M.; Domingues, M.; de Andrade Lourenço, D.; Birmann, P.T.; Padilha, N.; Vieira, B.; Begnini, K.; Seixas, F.K.; Collares, T.; Lenardão, E.J.; et al. Depression- and anxiogenic-like behaviors induced by lipopolysaccharide in mice are reversed by a selenium-containing indolyl compound: Behavioral, neurochemical and computational insights involving the serotonergic system. J. Psychiatr. Res. 2019, 115, 1–12.
- Dhiman, P.; Malik, N.; Khatkar, A. Exploration of umbelliferone based derivatives as potent MAO inhibitors: Dry vs. wet lab evaluation. Curr. Top. Med. Chem. 2019, 18, 1857–1871.
- Muraro, C.; Dalla Tiezza, M.; Pavan, C.; Ribaudo, G.; Zagotto, G.; Orian, L. Major depressive disorder and oxidative stress: In silico investigation of fluoxetine activity against ROS. Appl. Sci. 2019, 9, 3631.
- Bortoli, M.; Dalla Tiezza, M.; Muraro, C.; Pavan, C.; Ribaudo, G.; Rodighiero, A.; Tubaro, C.; Zagotto, G.; Orian, L. Psychiatric disorders and oxidative injury: Antioxidant effects of zolpidem therapy disclosed in silico. Comput. Struct. Biotechnol. J. 2019, 17, 311–318.
- Galano, A.; Raúl Alvarez-Idaboy, J. Computational strategies for predicting free radical scavengers’ protection against oxidative stress: Where are we and what might follow? Int. J. Quantum Chem. 2019, 119, e25665.
- Avram, S.; Borcan, F.; Borcan, L.-C.; Milac, A.L.; Mihailescu, D. QSAR approaches applied to antidepressants induced neurogenesis—In vivo and in silico applications. Mini-Rev. Med. Chem. 2015, 16, 230–240.
- Mernea, M.; Borcan, L.-C.; Borcan, F.; Avram, S. Antipsychotics as psychosis drugs and neuroprotective promoters evaluated by chemical QSAR—In silico and in vivo studies. Lett. Drug Des. Discov. 2016, 13, 269–275.
- Silva, D.R.; Barigye, S.J.; Santos-Garcia, L.; Fontes Ferreira da Cunha, E. Molecular modelling of potential candidates for the treatment of depression. Mol. Inform. 2019, 38, 1900024.
- Yan Zhao; Donald G. Truhlar; A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. The Journal of Chemical Physics 2006, 125, 194101, 10.1063/1.2370993.