Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
In 1987, bacteria were found to insert 32-nt (nucleotide) spacer sequences into 29-nt repeat sequences in CRISPR loci whenever they came into contact with phage DNA, leading to the discovery of the CRISPR-Cas system. The CRISPR-Cas-based technology can be employed as a new genome-editing tool in various organisms. The new gene-editing technique holds great promise for research and therapy of neurodegenerative diseases (NDs), such as Parkinson's disease (PD), for which there are currently no effective disease-modifying treatments.
- Parkinson’s
- CRISPR-Cas9
- gene therapy
- delivery
1. CRISPR-Cas
1.1. History
In 1987, bacteria were found to insert 32-nt (nucleotide) spacer sequences into 29-nt repeat sequences in CRISPR loci whenever they came into contact with phage DNA, leading to the discovery of the CRISPR-Cas system [1]. Similar repeating sequences were discovered in other E. coli strains: enterobacteria closely related to E. coli, and Shigella dysentery in the following years [2]. In 1993, Mojica and colleagues found the CRISPR repetitive sequence in archaea while researching the effects of salinity on the growth of Haloferax mediterranei. Although there was no similarity between these sequences and E.coli repeats, these researchers discovered a lengthy DNA sequence in the genome of these archaea that consisted of regulatory repeats [3]. In the CRISPR-Cas era, 2005 is regarded as a pivotal year because it was recognized that the spacer sequences were derived from phage genomes [4]. Together with the finding that Cas-gene encoded proteins with putative helicase and nuclease domains [5][6][7], and that CRISPR loci can be transcribed [8], It was recommended that CRISPR-cas is an adaptive system that may use antisense RNAs as a memory marker of past invasions [9]. In 2007, it was suggested that the CRISPR system could be used as an adaptive immune defense for bacteria and archaea against phage attacks. For example, adding or deleting spacer DNA homologous to phage DNA can alter the resistance of Streptococcus thermophilus to phage invasion [10]. In 2008, mature CRISPR RNAs (crRNAs) were determined to act as guides in a complex with Cas proteins in E. coli, preventing viral replication [11]. The CRISPR-Cas system’s DNA targeting activity was identified in the pathogen Staphylococcus epidermidis the same year. For nearly 20 years after their discovery, the function of these repeats remained unknown. Multiple direct repeats (DRs), short regulatory spaced repeats, and large clusters of tandem repeats have all been proposed as names for these repeats. Jansen and coworkers invented the word CRISPR, which has now gained acceptance among researchers since it reflects the structural properties of repeats [12][13][14][15].
1.2. CRISPR-Cas System
The classification of the CRISPR-Cas system is very challenging because there are no universal Cas proteins that could have served as phylogenetic markers. Consequently, the classification is based on many features, including the layout of Cas operons, signature Cas genes, and phylogenies of conserved Cas proteins [16]. There are two classes (Class 1 and Class 2), six types (I–VI), and 33 subtypes of CRISPR-Cas, according to a classification published in 2020 [17]. Multi-subunit effector complexes are seen in Class 1, while single protein effector modules are found in Class 2. Identifying two new types and several subtypes of the Class 2 CRISPR-Cas system resulted in more research and analysis of the system. The type VI systems, out of the two recently identified and defined CRISPR types, were the only ones that targeted RNA. In some circumstances, the class 2 systems have a unique feature in which the effector protein is also involved in processing pre-crRNA (CRISPR RNA) [18]. The CRISPR-Cas system’s two major classes, 1 and 2, have a solid basis of variation. The multi-subunit crRNA effector complex is classified as Class 1, while the single crRNA effector complex has been classified as Class 2. The Class 1 CRISPR-Cas system has been subdivided into types (I, III, and IV) and further into subtypes. Similarly, Class 2 is divided into three types: II, V, and VI, each further classified into multiple subtypes. The most widely used CRISP-Cas system is the type II CRISPR-Cas system which has been obtained from Streptococcus pyogens (SpCas9) [19][20]. The two main components of the CRISPR-Cas9 system are single guided RNA (sgRNA) and RNA guided Cas9 endonuclease [21]. There are two nuclease domains of Cas9, named RuvC and HNH, each breaking a single strand of targeted double-stranded DNA [22]. The RuvC domain cleaves the non-complimentary strand of dsDNA interacting with crRNA, while the HNH domain cuts the complementary strand [23]. A single-guide RNA (sgRNA) is a condensed form of crRNA and tracrRNA [24]. The Cas9 nuclease and sgRNA combine to form a Cas9 ribonucleoprotein (RNP) that can bind to and cleave the specific target in DNA [23].
Furthermore, the desired task of CRISPR-Cas9 systems is provided by the protospacer adjacent motif (PAM), which is an area inside an invading DNA that helps bacteria in differentiating pathogenic genetic information from its own [25][26]. If the spacer sequence is entirely identical to PAM, the CRISPR-Cas9 system will exclusively target plasmid or viral genetic materials by generating double-stranded (ds) DNA breaks in the invaded DNA [27]. As a result of these findings, researchers have determined that the CRISPR-Cas9 system can be employed as a new genome-editing tool in various organisms. It causes double-strand breaks (DSB), which can be fixed by either the homologous directed repair pathway (HDR) or error-prone non-homologous end junction (NHEJ) pathway, which are both endogenous self-healing processes [28]. NHEJ is more effective than HDR in most cases because it does not depend on a nearby homology donor and is also active for approximately 90% of the cell cycle [29]. NHEJ can integrate random insertion or deletion (indel) into the cleavage site, resulting in frameshift mutation or early termination codon in the open reading frame of the target gene so as to inactivate it [30][31]. However, HDR can introduce precise genomic changes at the target site using homologous DNA repair templates [32][33]. In addition, many sgRNAs targeting one or more genes can be used to create large deletions and knock out many genes at the same time [34][35].
2. Parkinson’s Disease
Parkinson’s disease (PD), after Alzheimer’s disease, is the second most common human neurodegenerative disease characterized by affected body movements [36]. PD is a heterogeneous neurodegenerative condition that affects an estimated 10 million people globally [37]. The progressive loss of dopaminergic neurons (DNs) in the substantia nigra pars compacta (SNpc) causes motor symptoms such as rest tremors, bradykinesia, and rigidity, which constitute the core of PD clinical characteristics [38]. This neuronal loss is followed by the appearance of cytoplasmic inclusions of Lewy bodies (LBs), which are primarily made of aggregates of misfolded α-synuclein protein and may spread in a prion-like way between synaptically interconnected areas [39] that has been supported in in vitro, in vivo and autopsy studies [40].
In addition, non-motor symptoms like cognitive decline, sleeping problems, depression, intestinal dysfunction, and anxiety are also becoming more commonly recognized as key factors in a patient’s standard of living and impairment [41]. PD prevalence rises with age (from 40–49 years up to people aged >80 years), and it is gender-dependent, with it being twice as common in males than in females [42][43]. The incidence rate of PD worldwide is increasing, and by 2040, the number of people suffering from the disease is expected to be close to 12 million, prompting some scholars to list it as a pandemic [44][45]. The majority of PD patients are classed as idiopathic, with approximately 10% having a proven monogenic cause (familial PD). Idiopathic PD’s etiology is unknown, but genetics, aging and environmental factors and their interactions have a role in the disease’s onset and development. Ninety common polymorphisms linked to the development of PD have been discovered in genome-wide studies [46][47], and the influence of genetic factors on the clinical heterogeneity and development of PD is still being investigated. Currently, the most common treatment for PD is symptomatic medication therapy. No mechanism-based treatment methods to prevent, regulate, or minimize the clinical signs of PD have been developed [48][49]. Additionally, the symptomatic therapeutic modalities used have many side effects. With the progression of the disease, the nonlinear pharmacodynamics of dopamine (DA) replacement therapy complicates the optimization of a treatment regimen [50]. A few cell replacement therapy researchers have demonstrated the feasibility of producing DNs from human embryonic stem cells (hESCs) and implanting these cells in animal PD models [51][52]. The early findings revealed that DA levels in the brains of experimental animals had increased [53]. However, this technique has several unresolved issues, including the possibility of immunologic response, brain tumors, ethical considerations, phenotype instability of hESC-derived DA neurons, and the need to assess the treatment’s effectiveness and safety in PD patients.
3. Application of CRISPR-Cas in PD
Based on the observation that α-synuclein accumulation in microglia induced severe neurodegeneration of DNs [54][55], vaccines against α-synuclein might be an effective treatment option. However, no research focused on this method has yet been published. New mechanistic studies are needed to better understand the pathogenesis of PD, in which environmental and genetic variables contribute to a range of aberrant metabolic pathways and incorrect interactions between different macromolecules. The CRISPR-Cas9 system—a revolutionary technology created in the last decade that allows for immediate and accurate genome editing in nearly any living species—seems to be a promising approach in PD also [56][57]. CRISPR-Cas9 offers the possibility to accelerate basic research, focusing on elucidating the pathogenicity of neurological diseases and leading to new therapies, according to several recent articles, mainly for PD [58][59]. CRISPR-Cas9 technology is more succinct, versatile, and cost-effective than other gene-editing methods, resulting in its increasing popularity [21]. The CRISPR-Cas9 system enables to edit candidate genes (Table 1) to generate appropriate animal and cell line models, significantly improving the understanding of the disease. In the future, it may become an important tool for effective and valuable gene therapy, which is considered to be a new therapeutic strategy for PD [60].
Table 1. Genes implicated in the development of PD, their loci, proteins, functions, phenotypes, and neuropathology.
| Genes | Gene Locus | Alternative Names of the Gene | Proteins | Gene Function | Results of Gene Mutation | Onset of PD | |
|---|---|---|---|---|---|---|---|
| PRKN | 6q26 | PARK2 | Parkin | Parkin is a 465-amino-acid cytosolic E3 ubiquitin ligase that participates in proteasome-mediated protein degradation. It damages misfolded and overproduced proteins, as well as ubiquitin. | The absence of LB, dopaminergic neuron apoptosis in the SN, and neurofibrillary | Early | [61][62][63][64][65][66] |
| SNCA | 4q22.1 | PARK 1/PARK 4 | α-synuclein | The SNCA gene produces a protein called -synuclein, widely distributed in neurons. Its function is unknown; however, it may be involved in regulating vesicular and dopamine neurotransmission. | The broad presence of LB throughout the brain and cerebral cortex, as well as neuronal destruction in the LC and SN | Early | [67][68][69] |
| PINK1 | 1p36.12 | PARK6 | PTEN induced putative kinase 1 | The mitochondrial function of this protein is to protect the mitochondria from the damaging effects of cellular oxidative stress. | The occurrence of LB in the reticular nuclei of the brainstem and neuronal loss in the SN pars compacta | Early | [70][71][72] |
| RAB39B | Xq28 | None | RAB proteins, like RAB39B | These are members of the GTPase family. RAB39B controls the movement of vesicles between membrane compartments. | Extensive dopaminergic neuron loss in SN and classical LB disorder | X-linked early-onset | [73][74][75] |
| D-J1 | 1p36.23 | PARK7 | DJ-1 | Several tissue and organs, including the brain, contain the DJ-1 protein. This protein acts as a chaperone molecule and prevents cells from oxidative stress. DJ-1 assists in the refolding of damaged proteins as well as the assembly of specific proteins into the right three-dimensional shape. | LB pathology | Early | [76][77][78][79] |
| LRRK2 | 12q12 | PARK8 | Leucine-rich repeat kinase 2 | The protein Roco family includes the component of the gene LRRK2. It is involved in cytoskeletal dynamics, autophagy, and vesicular transport. | Heterogeneous: degeneration of neurons in the SN and occurrence of LB in the brain; specific cases: Neurofibrillary tangle pathology, lack of LB, and neural nigral degeneration | Late | [80][81][82] |
PD, Parkinson’s disease; SNCA, Synuclein alpha; SN, substantia nigra; LB, Lewy body; LC, locus coeruleus; LRRK2, leucine-rich repeat kinase 2; PINK1, PTEN-induce kinase 1.
CRISPR-Cas9 technologies have been proposed to offer a number of genomic modifications in addition to site-directed gene editing. CRISPR interference (CRISPRi) and CRISPR activation (CRISPRa) technologies have also been used to regulate the expression of target genes by making precise base modifications with a catalytically dead nuclease (dCas9) [83][84][85][86]. In addition, they have been adapted as tools for gene location detection [87], epigenetic research [88], and even modified RNA targeting (Figure 1) [89].
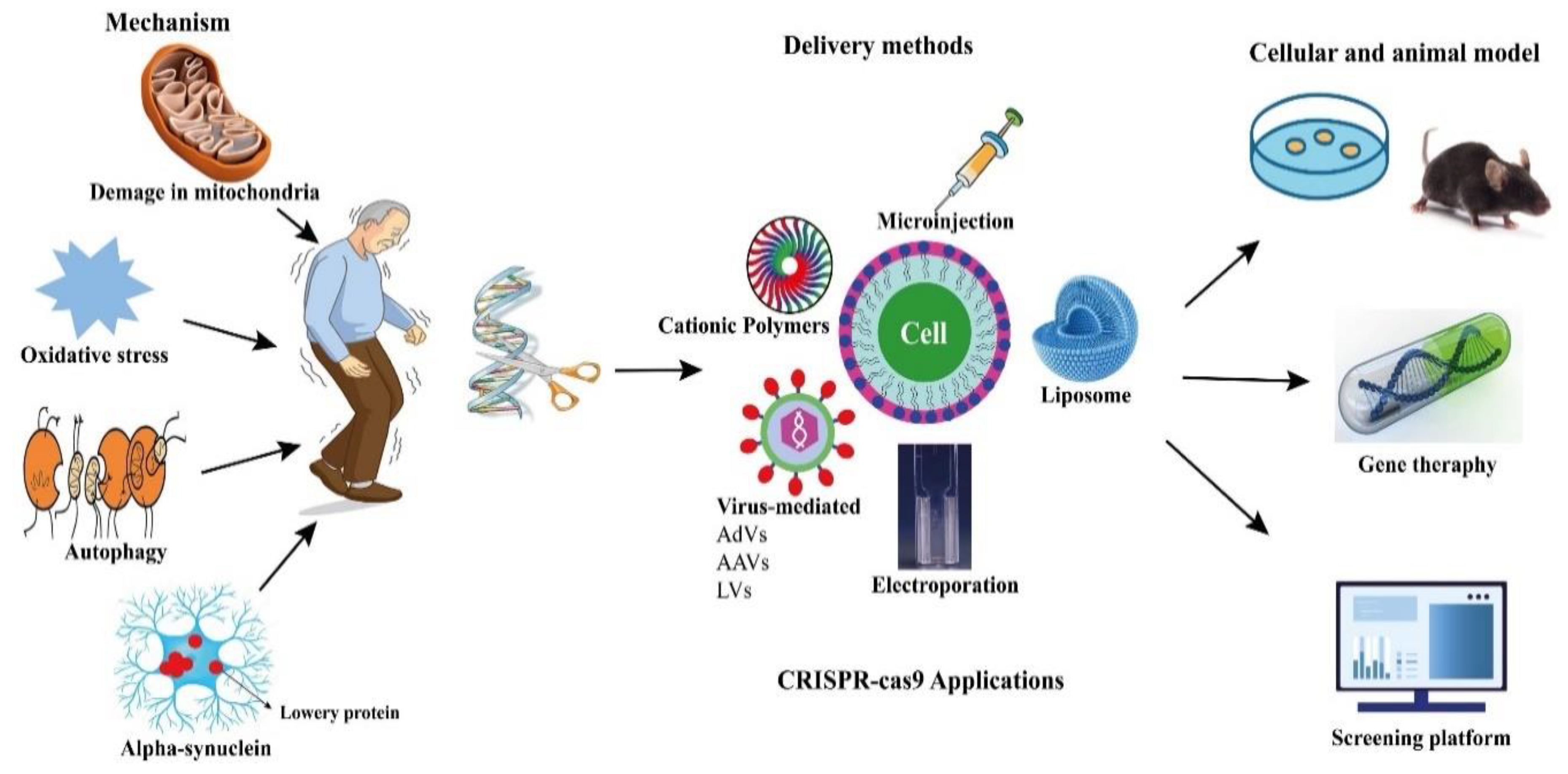 Figure 1. Potential applications of CRISPR-Cas9 in PD.
Figure 1. Potential applications of CRISPR-Cas9 in PD.This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics14061252
References
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J. Bacteriol. 1987, 169, 5429–5433.
- Nakata, A.; Amemura, M.; Makino, K. Unusual nucleotide arrangement with repeated sequences in the Escherichia coli K-12 chromosome. J. Bacteriol. 1989, 171, 3553–3556.
- Mojica, F.J.; Juez, G.; Rodriguez-Valera, F. Transcription at different salinities of Haloferax mediterranei sequences adjacent to partially modified PstI sites. Mol. Microbiol. 1993, 9, 613–621.
- Mojica, F.J.; Díez-Villaseñor, C.; García-Martínez, J.; Soria, E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J. Mol. Evol. 2005, 60, 174–182.
- Bolotin, A.; Quinquis, B.; Sorokin, A.; Ehrlich, S.D. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology 2005, 151, 2551–2561.
- Pourcel, C.; Salvignol, G.; Vergnaud, G. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology 2005, 151, 653–663.
- Haft, D.H.; Selengut, J.; Mongodin, E.F.; Nelson, K.E. A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Comput. Biol. 2005, 1, e60.
- Tang, T.-H.; Bachellerie, J.-P.; Rozhdestvensky, T.; Bortolin, M.-L.; Huber, H.; Drungowski, M.; Elge, T.; Brosius, J.; Hüttenhofer, A. Identification of 86 candidates for small non-messenger RNAs from the archaeon Archaeoglobus fulgidus. Proc. Natl. Acad. Sci. USA 2002, 99, 7536–7541.
- Makarova, K.S.; Grishin, N.V.; Shabalina, S.A.; Wolf, Y.I.; Koonin, E.V. A putative RNA-interference-based immune system in prokaryotes: Computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action. Biol. Direct 2006, 1, 7.
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR provides acquired resistance against viruses in prokaryotes. Science 2007, 315, 1709–1712.
- Brouns, S.J.; Jore, M.M.; Lundgren, M.; Westra, E.R.; Slijkhuis, R.J.; Snijders, A.P.; Dickman, M.J.; Makarova, K.S.; Koonin, E.V.; Van Der Oost, J. Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 2008, 321, 960–964.
- Groenen, P.M.; Bunschoten, A.E.; Soolingen, D.v.; Errtbden, J.D.v. Nature of DNA polymorphism in the direct repeat cluster of Mycobacterium tuberculosis; application for strain differentiation by a novel typing method. Mol. Microbiol. 1993, 10, 1057–1065.
- Mojica, F.J.; Díez-Villaseñor, C.; Soria, E.; Juez, G. Biological significance of a family of regularly spaced repeats in the genomes of Archaea, Bacteria and mitochondria. Mol. Microbiol. 2000, 36, 244–246.
- She, Q.; Singh, R.K.; Confalonieri, F.; Zivanovic, Y.; Allard, G.; Awayez, M.J.; Christina, C.-Y.; Clausen, I.G.; Curtis, B.A.; De Moors, A. The complete genome of the crenarchaeon Sulfolobus solfataricus P2. Proc. Natl. Acad. Sci. USA 2001, 98, 7835–7840.
- Jansen, R.; van Embden, J.D.; Gaastra, W.; Schouls, L.M. Identification of genes that are associated with DNA repeats in prokaryotes. Mol. Microbiol. 2002, 43, 1565–1575.
- Koonin, E.V.; Makarova, K.S. Origins and evolution of CRISPR-Cas systems. Philos. Trans. R. Soc. B 2019, 374, 20180087.
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P. Evolutionary classification of CRISPR–Cas systems: A burst of class 2 and derived variants. Nat. Rev. Microbiol. 2020, 18, 67–83.
- Koonin, E.V.; Makarova, K.S.; Zhang, F. Diversity, classification and evolution of CRISPR-Cas systems. Curr. Opin. Microbiol. 2017, 37, 67–78.
- Jiang, F.; Doudna, J.A. CRISPR–Cas9 structures and mechanisms. Annu. Rev. Biophys. 2017, 46, 505–529.
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823–826.
- Ran, F.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308.
- Chen, H.; Choi, J.; Bailey, S. Cut site selection by the two nuclease domains of the Cas9 RNA-guided endonuclease. J. Biol. Chem. 2014, 289, 13284–13294.
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA–guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821.
- Deltcheva, E.; Chylinski, K.; Sharma, C.M.; Gonzales, K.; Chao, Y.; Pirzada, Z.A.; Eckert, M.R.; Vogel, J.; Charpentier, E. CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature 2011, 471, 602–607.
- Mojica, F.J.; Díez-Villaseñor, C.; García-Martínez, J.; Almendros, C. Short motif sequences determine the targets of the prokaryotic CRISPR defence system. Microbiology 2009, 155, 733–740.
- Deveau, H.; Barrangou, R.; Garneau, J.E.; Labonté, J.; Fremaux, C.; Boyaval, P.; Romero, D.A.; Horvath, P.; Moineau, S. Phage response to CRISPR-encoded resistance in Streptococcus thermophilus. J. Bacteriol. 2008, 190, 1390–1400.
- Garneau, J.E.; Dupuis, M.-È.; Villion, M.; Romero, D.A.; Barrangou, R.; Boyaval, P.; Fremaux, C.; Horvath, P.; Magadán, A.H.; Moineau, S. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 2010, 468, 67–71.
- Ceccaldi, R.; Rondinelli, B.; D’Andrea, A.D. Repair pathway choices and consequences at the double-strand break. Trends Cell Biol. 2016, 26, 52–64.
- Patsali, P.; Kleanthous, M.; Lederer, C.W. Disruptive technology: CRISPR/Cas-based tools and approaches. Mol. Diagn. Ther. 2019, 23, 187–200.
- Weterings, E.; Chen, D.J. The endless tale of non-homologous end-joining. Cell Res. 2008, 18, 114–124.
- Lieber, M.R. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu. Rev. Biochem. 2010, 79, 181–211.
- San Filippo, J.; Sung, P.; Klein, H. Mechanism of eukaryotic homologous recombination. Annu. Rev. Biochem. 2008, 77, 229–257.
- Richardson, C.D.; Ray, G.J.; DeWitt, M.A.; Curie, G.L.; Corn, J.E. Enhancing homology-directed genome editing by catalytically active and inactive CRISPR-Cas9 using asymmetric donor DNA. Nat. Biotechnol. 2016, 34, 339–344.
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823.
- Minkenberg, B.; Wheatley, M.; Yang, Y. CRISPR/Cas9-enabled multiplex genome editing and its application. Prog. Mol. Biol. Transl. Sci. 2017, 149, 111–132.
- Troncoso-Escudero, P.; Sepulveda, D.; Pérez-Arancibia, R.; Parra, A.V.; Arcos, J.; Grunenwald, F.; Vidal, R.L. On the right track to treat movement disorders: Promising therapeutic approaches for Parkinson’s and Huntington’s disease. Front. Aging Neurosci. 2020, 12, 571185.
- Ball, N.; Teo, W.-P.; Chandra, S.; Chapman, J. Parkinson’s disease and the environment. Front. Neurol. 2019, 10, 218.
- Magrinelli, F.; Picelli, A.; Tocco, P.; Federico, A.; Roncari, L.; Smania, N.; Zanette, G.; Tamburin, S. Pathophysiology of motor dysfunction in Parkinson’s disease as the rationale for drug treatment and rehabilitation. Parkinson’s Dis. 2016, 2016, 9832839.
- Ma, J.; Gao, J.; Wang, J.; Xie, A. Prion-like mechanisms in Parkinson’s disease. Front. Neurosci. 2019, 13, 552.
- Kujawska, M.; Jodynis-Liebert, J. What is the Evidence That Parkinson’s Disease is a Prion Disorder, Which Originates in the Gut? Int. J. Mol. Sci. 2018, 19, 3573.
- Magnard, R.; Vachez, Y.; Carcenac, C.; Krack, P.; David, O.; Savasta, M.; Boulet, S.; Carnicella, S. What can rodent models tell us about apathy and associated neuropsychiatric symptoms in Parkinson’s disease? Transl. Psychiatry 2016, 6, e753.
- Van Den Eeden, S.K.; Tanner, C.M.; Bernstein, A.L.; Fross, R.D.; Leimpeter, A.; Bloch, D.A.; Nelson, L.M. Incidence of Parkinson’s disease: Variation by age, gender, and race/ethnicity. Am. J. Epidemiol. 2003, 157, 1015–1022.
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590.
- Dorsey, E.R.; Bloem, B.R. The Parkinson pandemic—A call to action. JAMA Neurol. 2018, 75, 9–10.
- Dorsey, E.; Sherer, T.; Okun, M.S.; Bloem, B.R. The emerging evidence of the Parkinson pandemic. J. Parkinson’s Dis. 2018, 8, S3–S8.
- Nalls, M.A.; Blauwendraat, C.; Vallerga, C.L.; Heilbron, K.; Bandres-Ciga, S.; Chang, D.; Tan, M.; Kia, D.A.; Noyce, A.J.; Xue, A. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 2019, 18, 1091–1102.
- Keller, M.F.; Saad, M.; Bras, J.; Bettella, F.; Nicolaou, N.; Simón-Sánchez, J.; Mittag, F.; Büchel, F.; Sharma, M.; Gibbs, J.R. Using genome-wide complex trait analysis to quantify ‘missing heritability’in Parkinson’s disease. Hum. Mol. Genet. 2012, 21, 4996–5009.
- Abeliovich, A.; Gitler, A.D. Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature 2016, 539, 207–216.
- Connolly, B.S.; Lang, A.E. Pharmacological treatment of Parkinson disease: A review. JAMA 2014, 311, 1670–1683.
- Kujawska, M.; Bhardwaj, S.K.; Mishra, Y.K.; Kaushik, A. Using Graphene-Based Biosensors to Detect Dopamine for Efficient Parkinson’s Disease Diagnostics. Biosensors 2021, 11, 433.
- Grealish, S.; Diguet, E.; Kirkeby, A.; Mattsson, B.; Heuer, A.; Bramoulle, Y.; Van Camp, N.; Perrier, A.L.; Hantraye, P.; Björklund, A. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson’s disease. Cell Stem Cell 2014, 15, 653–665.
- Wang, Y.-K.; Zhu, W.-W.; Wu, M.-H.; Wu, Y.-H.; Liu, Z.-X.; Liang, L.-M.; Sheng, C.; Hao, J.; Wang, L.; Li, W. Human clinical-grade parthenogenetic ESC-derived dopaminergic neurons recover locomotive defects of nonhuman primate models of Parkinson’s disease. Stem Cell Rep. 2018, 11, 171–182.
- Kriks, S.; Shim, J.-W.; Piao, J.; Ganat, Y.M.; Wakeman, D.R.; Xie, Z.; Carrillo-Reid, L.; Auyeung, G.; Antonacci, C.; Buch, A. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 2011, 480, 547–551.
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369.
- Kujawska, M.; Domanskyi, A.; Kreiner, G. Editorial: Common Pathways Linking Neurodegenerative Diseases—The Role of Inflammation. Front. Cell. Neurosci. 2021, 15, 754051.
- Adli, M. The CRISPR tool kit for genome editing and beyond. Nat. Commun. 2018, 9, 1911.
- Sander, J.D.; Joung, J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014, 32, 347–355.
- Kantor, B.; Tagliafierro, L.; Gu, J.; Zamora, M.E.; Ilich, E.; Grenier, C.; Huang, Z.Y.; Murphy, S.; Chiba-Falek, O. Downregulation of SNCA expression by targeted editing of DNA methylation: A potential strategy for precision therapy in PD. Mol. Ther. 2018, 26, 2638–2649.
- Kim, S.; Yun, S.P.; Lee, S.; Umanah, G.E.; Bandaru, V.V.R.; Yin, X.; Rhee, P.; Karuppagounder, S.S.; Kwon, S.-H.; Lee, H. GBA1 deficiency negatively affects physiological α-synuclein tetramers and related multimers. Proc. Natl. Acad. Sci. USA 2018, 115, 798–803.
- Wu, S.-S.; Li, Q.-C.; Yin, C.-Q.; Xue, W.; Song, C.-Q. Advances in CRISPR/Cas-based gene therapy in human genetic diseases. Theranostics 2020, 10, 4374.
- Zhang, Y.; Gao, J.; Chung, K.K.; Huang, H.; Dawson, V.L.; Dawson, T.M. Parkin functions as an E2-dependent ubiquitin–protein ligase and promotes the degradation of the synaptic vesicle-associated protein, CDCrel-1. Proc. Natl. Acad. Sci. USA 2000, 97, 13354–13359.
- Bradshaw, A.V.; Campbell, P.; Schapira, A.H.; Morris, H.R.; Taanman, J.-W. The PINK1—Parkin mitophagy signalling pathway is not functional in peripheral blood mononuclear cells. PLoS ONE 2021, 16, e0259903.
- Geisler, S.; Holmström, K.M.; Treis, A.; Skujat, D.; Weber, S.S.; Fiesel, F.C.; Kahle, P.J.; Springer, W. The PINK1/Parkin-mediated mitophagy is compromised by PD-associated mutations. Autophagy 2010, 6, 871–878.
- Kitada, T.; Asakawa, S.; Hattori, N.; Matsumine, H.; Yamamura, Y.; Minoshima, S.; Yokochi, M.; Mizuno, Y.; Shimizu, N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 1998, 392, 605–608.
- Gouider-Khouja, N.; Larnaout, A.; Amouri, R.; Sfar, S.; Belal, S.; Hamida, C.B.; Hamida, M.B.; Hattori, N.; Mizuno, Y.; Hentati, F. Autosomal recessive parkinsonism linked to parkin gene in a Tunisian family. Clinical, genetic and pathological study. Parkinsonism Relat. Disord. 2003, 9, 247–251.
- Miyakawa, S.; Ogino, M.; Funabe, S.; Uchino, A.; Shimo, Y.; Hattori, N.; Ichinoe, M.; Mikami, T.; Saegusa, M.; Nishiyama, K. Lewy body pathology in a patient with a homozygous parkin deletion. Mov. Disord. 2013, 28, 388–391.
- Polymeropoulos, M.H.; Higgins, J.J.; Golbe, L.I.; Johnson, W.G.; Ide, S.E.; Di Iorio, G.; Sanges, G.; Stenroos, E.S.; Pho, L.T.; Schaffer, A.A. Mapping of a gene for Parkinson’s disease to chromosome 4q21-q23. Science 1996, 274, 1197–1199.
- Klucken, J.; Ingelsson, M.; Shin, Y.; Irizarry, M.C.; Hedley-Whyte, E.; Frosch, M.P.; Growdon, J.H.; McLean, P.J.; Hyman, B.T. Clinical and biochemical correlates of insoluble α-synuclein in dementia with Lewy bodies. Acta Neuropathol. 2006, 111, 101–108.
- Ellis, C.E.; Murphy, E.J.; Mitchell, D.C.; Golovko, M.Y.; Scaglia, F.; Barceló-Coblijn, G.C.; Nussbaum, R.L. Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking α-synuclein. Mol. Cell. Biol. 2005, 25, 10190–10201.
- Samaranch, L.; Lorenzo-Betancor, O.; Arbelo, J.M.; Ferrer, I.; Lorenzo, E.; Irigoyen, J.; Pastor, M.A.; Marrero, C.; Isla, C.; Herrera-Henriquez, J. PINK1-linked parkinsonism is associated with Lewy body pathology. Brain 2010, 133, 1128–1142.
- Sliter, D.A.; Martinez, J.; Hao, L.; Chen, X.; Sun, N.; Fischer, T.D.; Burman, J.L.; Li, Y.; Zhang, Z.; Narendra, D.P. Parkin and PINK1 mitigate STING-induced inflammation. Nature 2018, 561, 258–262.
- Valente, E.M.; Abou-Sleiman, P.M.; Caputo, V.; Muqit, M.M.; Harvey, K.; Gispert, S.; Ali, Z.; Del Turco, D.; Bentivoglio, A.R.; Healy, D.G. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 2004, 304, 1158–1160.
- Cheng, H.; Ma, Y.; Ni, X.; Jiang, M.; Guo, L.; Ying, K.; Xie, Y.; Mao, Y. Isolation and characterization of a human novel RAB (RAB39B) gene. Cytogenet. Genome Res. 2002, 97, 72–75.
- Wilson, G.R.; Sim, J.C.; McLean, C.; Giannandrea, M.; Galea, C.A.; Riseley, J.R.; Stephenson, S.E.; Fitzpatrick, E.; Haas, S.A.; Pope, K. Mutations in RAB39B cause X-linked intellectual disability and early-onset Parkinson disease with α-synuclein pathology. Am. J. Hum. Genet. 2014, 95, 729–735.
- Lesage, S.; Bras, J.; Cormier-Dequaire, F.; Condroyer, C.; Nicolas, A.; Darwent, L.; Guerreiro, R.; Majounie, E.; Federoff, M.; Heutink, P. Loss-of-function mutations in RAB39B are associated with typical early-onset Parkinson disease. Neurol. Genet. 2015, 1, e9.
- Annesi, G.; Savettieri, G.; Pugliese, P.; D’Amelio, M.; Tarantino, P.; Ragonese, P.; La Bella, V.; Piccoli, T.; Civitelli, D.; Annesi, F. DJ-1 mutations and parkinsonism-dementia-amyotrophic lateral sclerosis complex. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2005, 58, 803–807.
- Abou-Sleiman, P.M.; Healy, D.G.; Quinn, N.; Lees, A.J.; Wood, N.W. The role of pathogenic DJ-1 mutations in Parkinson’s disease. Ann. Neurol. 2003, 54, 283–286.
- Zhu, M.; Patel, S.H.; Han, S. DJ-1, a Parkinson’s disease related protein, aggregates under denaturing conditions and co-aggregates with α-synuclein through hydrophobic interaction. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 1759–1769.
- Taipa, R.; Pereira, C.; Reis, I.; Alonso, I.; Bastos-Lima, A.; Melo-Pires, M.; Magalhaes, M. DJ-1 linked parkinsonism (PARK7) is associated with Lewy body pathology. Brain 2016, 139, 1680–1687.
- Marín, I. Ancient origin of the Parkinson disease gene LRRK2. J. Mol. Evol. 2008, 67, 41–50.
- Healy, D.G.; Falchi, M.; O’Sullivan, S.S.; Bonifati, V.; Durr, A.; Bressman, S.; Brice, A.; Aasly, J.; Zabetian, C.P.; Goldwurm, S. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: A case-control study. Lancet Neurol. 2008, 7, 583–590.
- Zimprich, A.; Biskup, S.; Leitner, P.; Lichtner, P.; Farrer, M.; Lincoln, S.; Kachergus, J.; Hulihan, M.; Uitti, R.J.; Calne, D.B. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 2004, 44, 601–607.
- Qi, L.S.; Larson, M.H.; Gilbert, L.A.; Doudna, J.A.; Weissman, J.S.; Arkin, A.P.; Lim, W.A. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 2013, 152, 1173–1183.
- Tanenbaum, M.E.; Gilbert, L.A.; Qi, L.S.; Weissman, J.S.; Vale, R.D. A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell 2014, 159, 635–646.
- Gaudelli, N.M.; Komor, A.C.; Rees, H.A.; Packer, M.S.; Badran, A.H.; Bryson, D.I.; Liu, D.R. Programmable base editing of A• T to G• C in genomic DNA without DNA cleavage. Nature 2017, 551, 464–471.
- Kampmann, M. CRISPRi and CRISPRa screens in mammalian cells for precision biology and medicine. ACS Chem. Biol. 2018, 13, 406–416.
- Deng, W.; Shi, X.; Tjian, R.; Lionnet, T.; Singer, R.H. CASFISH: CRISPR/Cas9-mediated in situ labeling of genomic loci in fixed cells. Proc. Natl. Acad. Sci. USA 2015, 112, 11870–11875.
- Kang, J.G.; Park, J.S.; Ko, J.-H.; Kim, Y.-S. Regulation of gene expression by altered promoter methylation using a CRISPR/Cas9-mediated epigenetic editing system. Sci. Rep. 2019, 9, 11960.
- Ye, R.; Pi, M.; Cox, J.V.; Nishimoto, S.K.; Quarles, L.D. CRISPR/Cas9 targeting of GPRC6A suppresses prostate cancer tumorigenesis in a human xenograft model. J. Exp. Clin. Cancer Res. 2017, 36, 90.
This entry is offline, you can click here to edit this entry!
