Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Despite the popularity of the ginseng (Panax) root in health research and on the market, the ginseng berry’s potential remains relatively unexplored. Implementing ginseng berry cultivations and designing berry-derived products could improve the accessibility to mental health-promoting nutraceuticals.
- ginseng berry
- ginsenosides
- mental health
- neuroprotection
- nutraceutical
1. Introduction
Mental illness is debilitating and compromises the individual’s quality of life, as well as it has surprisingly far-reaching economic effects, costing the Canadian economy an estimated 51 billion dollars annually [1]. Globally, mental illness has been estimated to be responsible for 32.4% of years lived with disability, surpassing all other forms of diseases [2]. Mood and anxiety disorders are the most common mental illnesses globally and in Canada [3], where their estimated prevalence is 4.7% annually [4]. Unfortunately, many standard pharmaceutical treatments, such as antidepressants, have significant side effects that could affect adherence to the treatment, as well as mixed results with regards to efficacy [5]. Furthermore, it has recently been shown that antidepressant use could negatively impact the intestinal microbiota diversity and be detrimental to certain types of beneficial bacteria [6][7]. With the concept and applications of the microbiota–gut–brain axis gaining traction among the scientific community, complementary treatments targeting this axis to promote mental health are needed.
Ginseng, one of the most important herbs of traditional Chinese medicine, has an impressive track record of positive effects in both in vitro and in vivo models of mental health [8], while also displaying efficacy in clinical research [9][10][11]. However, in traditional Chinese medicine and even in modern research, the root has been the primary focus of health allegations and, by extension, the focus of ginseng culture. The berries, which are largely regarded as by-products of the ginseng root culture, have great potential for applications in health due to their pharmacological properties and distinct composition with respect to the root [12]. Despite the rationale strongly supporting the pharmacological properties of the berries [12], they remain underutilized and are frequently discarded in agriculture, while the root is marketed. As ginseng is a slowly growing crop, root cultures take multiple years to harvest. Furthermore, significant crop mortality following the replanting of new ginseng is an issue plaguing agriculture; thus, bioremedial efforts have been undertaken to mitigate this effect [13]. The berry culture, on the other hand, presents numerous advantages. For instance, berries can be harvested from the same ginseng plant annually starting on the second year of growth, without any detriment to the crop. The root culture, in contrast, takes 4 to 10 years to achieve a minimal marketable maturity. Finally, the berry culture can be implemented without impacting the current root harvesting practices.
The pharmacological mechanisms through the gut–brain axis in which ginseng could promote mental health, as shown in Figure 1.
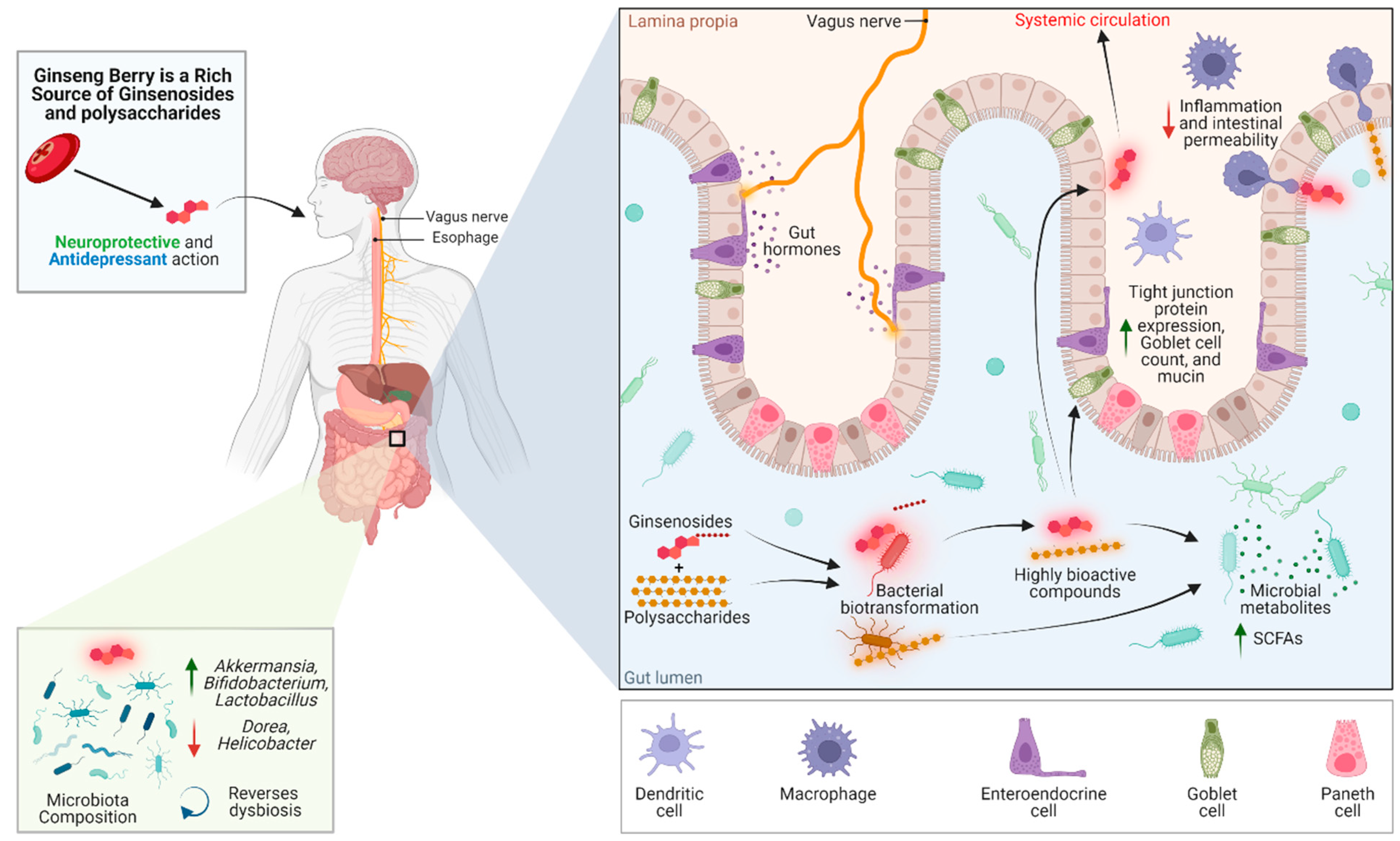
Figure 1. An overview of the reported studies highlighting the interplay of ginseng berry compounds with the microbiome–gut–brain axis. Green upward arrows represent a significant increase, whereas red downward arrows represent a significant decrease. Green arrow: increase; red arrow: decrease.
2. The Berry Is a Highly Concentrated Source of Ginseng’s Therapeutic Compounds
The main bioactive compounds in the berry are of the same classes as those found in the root. Ginsenosides, usually denoted by a capital R followed by a lowercase letter and a number, if required (e.g., Rg1), are saponins present in an impressive diversity within the same type of ginseng and even in the same part of the plant. Their structural diversity is naturally accompanied by a diverse range of pharmacological functions and efficacy. Ginseng root polysaccharide extracts have also been researched in various contexts. As shown in Table 1, the berry could contain higher levels of neuroprotective and antidepressant bioactive compounds than the root.
Table 1. A review of American ginseng berry bioactive compounds—(*B>) content is significantly higher in the berry than root, (*B<) content is significantly less than the root, (B~) content is not statistically different from the root, (nil) was not detected in the berry, (ND) not determined in the study. Significance was determined using a two-tailed t-test with p < 0.05. When multiple harvest times were available in a study, the harvest date closest to August 30th was chosen.
| Compounds | Pharmacological Effects | Content (Berry vs. Root, mg/g Dry Weight) |
|
|---|---|---|---|
| Ginsenosides | Rb1 | Neuroprotective [14], anti-diabetic [15], mitochondrial antioxidant [16] | *B< [12][17] 0.86 ± 0.09 vs. 25.36 ± 1.67 [17] 9.03 ± 0.60 vs. ND [18] ND vs. 48.51 ± 1.79 [19] ND vs. 47.96 ± 1.04 [19] |
| Rb2 | Anti-diabetic, anti-viral, cardioprotective, neuroprotective [20] | *B> [12][17][21] 1.54 ± 0.95 vs. 0.3 ± 0.02 [17] |
|
| Rb3 | Anti-diabetic, anticonvulsant, antitumor, cardioprotective, antidepressant [22] | *B> [12][21] | |
| Rc | Antiallergic [23], antioxidant [24], anti-inflammatory [25], SIRT1 activation [26] | *B> [12] *B< [17] 1.51 ± 0.11 vs. 7.03 ± 2.15 [17] |
|
| Rd | Neuroprotective, antioxidant, anti-inflammatory, neuroprotective [27], antidepressant [28] | B~ [12] *B< [17] 0.48 ± 0.1 vs. 3.16 ± 0.98 [17] |
|
| Re | Cardioprotective [29], Neuroprotective [30][31], antidepressant [32] | *B> [12] *B< [17] 5.30 ± 0.54 vs. 17.45 ± 1.6 [17] 8.42 ± 0.19 vs. ND [18] |
|
| Rg1 | Stem cell regulation [33][34], anti-inflammatory [35], antidepressant [36] | *B< [12][17] 0.53 ± 0.09 vs. 2.39 ± 1.01 [17] 0.390 ± 0.010 vs. ND [18] ND vs. 3.15 ± 0.23 [19] ND vs. 2.49 ± 0.04 [19] |
|
| Rg2 | Cardioprotective [37][38][39][40], neuroprotective [41] | *B> [12] | |
| 20(R)-Rg2 | Insufficient data | nil [12][42] | |
| Rg3 | Anticancer [43][44], neuroprotective [45] | *B> [12] | |
| Rh1 | Anti-inflammatory, antioxidant, immunomodulatory, neuroprotective [46] | B~ [12] | |
| Rh2 | Anti-cancer [47] | nil [12][42] | |
| Polysaccharides | Anti-cancer [48][49] | ||
It is reported that the total ginsenoside content could be higher in the berry than in the root by as much as 60% [12], though this is not consistent throughout the studies, perhaps due to varying harvest times and differences in the way that ginsenosides are measured in each study (e.g., measuring the root’s main ginsenosides but not the berry’s biases the total ginsenoside count). Rb3, Re, Rb2, Rd, and Rc, in descending order of abundance (with occasional variation between Re and Rb2), are the ginsenosides that are the most abundant in the American ginseng berry, and this is consistent throughout the studies assessing its composition via high-performance liquid chromatography [12][42][50][51]. Of note, the berry ginsenoside content can be different depending on the variety of ginseng selected. For instance, ginsenoside Re is the most abundant in Korean ginseng berries and is approximately 8 times more concentrated than Rb2 [52], whereas in the American ginseng, Re is at most 1.2 times as concentrated as Rb2 [12]. The harvest time has also been shown to cause significant variance in ginsenoside content; American ginseng berries were shown to lose over half of their Rb1, Re, and Rg1 content during the season, strongly suggesting that the ginsenoside content may be at its peak before the berries are ripe [18]. Post-harvest treatment should also be considered, as steaming has been shown to cause a sharp decrease in the total content, consistently causing a loss of about 50% after 2 h of steaming at 120 °C [42][50]. Conversely, ginsenosides Rh1, Rg2, (20)R-Rg2, Rg3, and Rh2 sharply increased in content after a 2 h steaming treatment [42][50].
Given the berry’s high concentration of Rb3, Re, Rb2, and Rd, the ginsenosides with demonstrated antidepressant and neuroprotective effects [20][22][27][30][31], it could be expected that the berry has even a superior potential for mental health applications than the root. Still, root extracts and specific ginsenosides have been the subject of most research and have consistently demonstrated efficacy in vitro and in vivo models in the context of central nervous system diseases and depression [53][54]. Another aspect to consider when evaluating the berry’s antidepressant and neuroprotective potential is that the microbial community of the intestine metabolizes the ginsenosides into alternate forms with varying effects and degrees of bioactivity. For instance, Rb3, the berry’s main ginsenoside, and its deglycosylated metabolites Rg3, Rh2, compound K, and 20(S)-protopanaxadiol have had their antidepressant potential assessed, and it was shown that Rg3 and compound K have more powerful antidepressant effects which are brought upon by the modulation of corticosterone, adrenocorticotropic hormone, and noradrenaline levels [55]. Thus, the fact that the berry has an inherently higher concentration of Rg3 than the root and a higher concentration of Rb3, which can, in turn, be deglycosylated into Rg3 [12][55], is a fine example of the berry’s untapped potential as a mental health-promoting nutraceutical.
3. Pharmacological Effects in The Context of Mental Health
Most of the mental health-promoting effects attributed to the berry come from extrapolation of data from single ginsenoside or total ginsenoside extract experiments. Data from experiments directly involving the berry or its distinct ginsenoside composition are scarce in the context of mental health. The berry saponin extract was shown to regulate 5-HT and rescue depressive-like behaviour in a mouse model of myocardial infarction, though this was fruit from the Panax notoginseng [56]. In fact, ginseng berry experiments with application to mental health seem to be limited to the examination of serotonin regulation in comorbid myocardial infarction models [57][58] and one additional study involving scopolamine-induced memory impairment, where the berry extract was shown to have antioxidant effects and to preserve acetylcholine and brain-derived neurotrophic factor (BDNF) mRNA levels [59]. This section illustrates the current mental health-related findings for the predominant ginsenosides in the American ginseng berry.
3.1. Ginsenoside Rb3
Rb3, the berry’s most abundant ginsenoside, exerts pharmacological effects that benefit mental health through multiple mechanisms. Such neuroprotective mechanisms occur through varied antioxidant effects, such as suppressing inducible nitric oxide synthase in hypoxic hippocampal neurons [60], preserving superoxide dismutase (SOD) and catalase (CAT) levels [61], and inducing Nrf2 transcription activity [62], which is downregulated in neurological conditions, such as depression [63]. Likewise, ginsenoside Rb3 was shown to interact with multiple neurotransmitters and receptors, leading to neuroprotective effects through inhibiting the NMDA receptor [64][65], activating the GABA(A) receptor [66], or acting beneficially on the noradrenergic pathway to relieve depression in rodent models [55][67].
3.2. Ginsenoside Re
Ginsenoside Re also demonstrates neuroprotective effects. It could be effective at reducing neuroinflammation by inhibiting the CAMK/MAPK/NF-κB signaling, as demonstrated by Madhi et al. [30], as well as by attenuating NLRP3 activation, as reported by Wang et al. [31]. In the same study, ginsenoside Re was also able to counter the loss of the antioxidant enzymes SOD, CAT, and glutathione (GSH) and the loss of Nrf2 expression following chronic restraint stress [31]. The compound also induced the expression of genes involved in acetylcholine neurotransmission, elevated acetylcholine levels, and enhanced the differentiation of Neuro-2a cells, which could translate to benefit in Alzheimer’s disease [68]. The neuronal effects also extend to reversing the depression- and anxiety-associated behavioural changes in rat models of repeated immobilization [32] and the learning and memory decline caused by chronic restraint in mice [31], while exerting BDNF-protecting effects in both studies.
3.3. Ginsenoside Rb2
Research evaluating the efficacy of ginsenoside Rb2 is scarce in the context of mental health, though it has been shown to protect against glutamate-mediated neurotoxicity in HT22 hippocampal cells [69]. Miao et al. have recently written a review compiling the pharmacological effects of Rb2, which include inhibition of oxidative stress, inflammation, and apoptosis through multiple pathways [20]. Although these effects were not tested in neurological models, some described pathways (SIRT1, AMPK, MAPK, and NF-κB) are relevant for many neurological conditions.
3.4. Ginsenoside Rd
Chen et al. have written a comprehensive review thoroughly describing the neuroprotective mechanisms of ginsenoside Rd [27]. Some key reported data include anti-inflammatory effects via the regulation of iNOS, COX-2, MAPK, and NF-κB, antioxidant effects through increasing the SOD, GSH, and CAT, and antiapoptotic effects in several models of neuron stress [27]. Ginsenoside Rd was more recently shown to exert a significant antidepressant effect in the chronic unpredictable mild stress and behavioural despair mouse models via the hypoxia-inducible factor-1α and to increase the expression of SYN1 and PSD 95, two synaptic plasticity-related proteins [28]. Also of note, ginsenoside Rd alleviated both Escherichia coli K1-induced colitis and depression/anxiety in mice as measured by light/dark transition, forced swimming, and tail suspension tests, while significantly countering induced IL-6 expression in plasma and NF-κB activation (both colonic and hippocampal) [70]. In the same study, ginsenoside also protected the hippocampal BDNF levels and even reversed some changes in intestinal microbiota, brought upon by the administration of Escherichia coli K1 [70].
Ginsenosides could also exert neuroprotective effects through the modulation of microRNA, and Rd modulating miR-144-5p in a glioblastoma model is one such example [71]. Rd upregulated miR-144-5p, which decreased both TLR2 and the proliferation of the glioblastoma cells [71]. Although it remains to be confirmed that ginsenoside Rd could systemically upregulate miR-144-5p in vivo at a significant level, by extrapolating this microRNA’s targets to other models, it could be hypothesized that ginsenoside Rd has the potential to act therapeutically where TLR2 antagonism has shown benefit. For instance, anti-TLR2 has proven beneficial in decreasing α-synuclein accumulation in neuronal and astroglia cells in Parkinson’s and dementia with Lewy bodies mouse models, accompanied by decreased neuroinflammation and behavioural deficits [72]. Notably, miR-144-5p has been downregulated in depression and anxiety relative to healthy controls and inversely correlated with depression scores [73]. Similarly, a psychological treatment that decreased depression scores decreased specific inflammation-associated proteins and increased miR-144-5p in another cohort of depression, anxiety, and stress-related disorder patients [74]. Recently, Hyun compiled research demonstrating the microRNA modulating effects of various ginsenosides [75], but it may be too early to further extend these findings to the context of mental health. As microRNAs continue to gain traction as therapeutic targets, more research evaluating ginseng’s ability to modulate microRNAs would be of benefit to the scientific community.
In summary, the American ginseng berry’s main ginsenosides are promising mental health-promoting compounds through multiple neuroprotective and anti-depressive mechanisms. As illustrated by a previously mentioned study involving E. coli K1 administration [70], the ginseng berry’s bioactive components can additionally exert mental health benefits by modulating microbiota and other intestinal health parameters.
This entry is adapted from the peer-reviewed paper 10.3390/nu14122523
References
- Lim, K.L.; Jacobs, P.; Ohinmaa, A.; Schopflocher, D.; Dewa, C.S. A New Population-Based Measure of the Economic Burden of Mental Illness in Canada. Chronic Dis. Can. 2008, 28, 92–98.
- Vigo, D.; Thornicroft, G.; Atun, R. Estimating the True Global Burden of Mental Illness. Lancet Psychiatry 2016, 3, 171–178.
- Public Health Agency of Canada Report from the Canadian Chronic Disease Surveillance System: Mood and Anxiety Disorders in Canada. 2016. Available online: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/report-canadian-chronic-disease-surveillance-system-mood-anxiety-disorders-canada-2016.html (accessed on 29 September 2021).
- Lam, R.W.; McIntosh, D.; Wang, J.; Enns, M.W.; Kolivakis, T.; Michalak, E.E.; Sareen, J.; Song, W.-Y.; Kennedy, S.H.; MacQueen, G.M.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder. Can. J. Psychiatry 2016, 61, 510–523.
- Almohammed, O.A.; Alsalem, A.A.; Almangour, A.A.; Alotaibi, L.H.; Al Yami, M.S.; Lai, L. Antidepressants and Health-Related Quality of Life (HRQoL) for Patients with Depression: Analysis of the Medical Expenditure Panel Survey from the United States. PLoS ONE 2022, 17, e0265928.
- Ait Chait, Y.; Mottawea, W.; Tompkins, T.A.; Hammami, R. Unravelling the Antimicrobial Action of Antidepressants on Gut Commensal Microbes. Sci. Rep. 2020, 10, 17878.
- Vich Vila, A.; Collij, V.; Sanna, S.; Sinha, T.; Imhann, F.; Bourgonje, A.R.; Mujagic, Z.; Jonkers, D.M.A.E.; Masclee, A.A.M.; Fu, J.; et al. Impact of Commonly Used Drugs on the Composition and Metabolic Function of the Gut Microbiota. Nat. Commun. 2020, 11, 362.
- Kim, Y.; Cho, S.-H. The Effect of Ginsenosides on Depression in Preclinical Studies: A Systematic Review and Meta-Analysis. J. Ginseng Res. 2021, 45, 420–432.
- Braz, A.S.; Morais, L.C.S.; Paula, A.P.; Diniz, M.F.F.M.; Almeida, R.N. Effects of Panax Ginseng Extract in Patients with Fibromyalgia: A 12-Week, Randomized, Double-Blind, Placebo-Controlled Trial. Rev. Bras. Psiquiatr. 2013, 35, 21–28.
- Jeong, H.-G.; Ko, Y.-H.; Oh, S.-Y.; Han, C.; Kim, T.; Joe, S.-H. Effect of Korean Red Ginseng as an Adjuvant Treatment for Women with Residual Symptoms of Major Depression. Asia-Pac. Psychiatry 2015, 7, 330–336.
- Lee, K.J.; Ji, G.E. The Effect of Fermented Red Ginseng on Depression Is Mediated by Lipids. Nutr. Neurosci. 2014, 17, 7–15.
- Wang, C.-Z.; Wu, J.A.; McEntee, E.; Yuan, C.-S. Saponins Composition in American Ginseng Leaf and Berry Assayed by High-Performance Liquid Chromatography. J. Agric. Food Chem. 2006, 54, 2261–2266.
- Dong, L.; Xu, J.; Zhang, L.; Cheng, R.; Wei, G.; Su, H.; Yang, J.; Qian, J.; Xu, R.; Chen, S. Rhizospheric Microbial Communities Are Driven by Panax Ginseng at Different Growth Stages and Biocontrol Bacteria Alleviates Replanting Mortality. Acta Pharm. Sin. B 2018, 8, 272–282.
- Ahmed, T.; Raza, S.H.; Maryam, A.; Setzer, W.N.; Braidy, N.; Nabavi, S.F.; de Oliveira, M.R.; Nabavi, S.M. Ginsenoside Rb1 as a Neuroprotective Agent: A Review. Brain Res. Bull. 2016, 125, 30–43.
- Zhou, P.; Xie, W.; He, S.; Sun, Y.; Meng, X.; Sun, G.; Sun, X. Ginsenoside Rb1 as an Anti-Diabetic Agent and Its Underlying Mechanism Analysis. Cells 2019, 8, 204.
- Zhou, P.; Xie, W.; Sun, Y.; Dai, Z.; Li, G.; Sun, G.; Sun, X. Ginsenoside Rb1 and Mitochondria: A Short Review of the Literature. Mol. Cell. Probes 2019, 43, 1–5.
- Kochan, E.; Kołodziej, B.; Gadomska, G.; Chmiel, A. Ginsenoside Contents in Panax Quinquefolium Organs from Field Cultivation. Z. Für Nat. C 2008, 63, 91–95.
- Sritularak, B.; Morinaga, O.; Yuan, C.-S.; Shoyama, Y.; Tanaka, H. Quantitative Analysis of Ginsenosides Rb1, Rg1, and Re in American Ginseng Berry and Flower Samples by ELISA Using Monoclonal Antibodies. J. Nat. Med. 2009, 63, 360–363.
- Son, T.; Eguchi, T.; Shoyama, Y.; Tanaka, H. ELISA for the Detection of Marker Compound for Crop Fertilizer Use of Various Medicinal Crop Extracts Using Bacterium. J.-Fac. Agric. Kyushu Univ. 2019, 64, 27–32.
- Miao, L.; Yang, Y.; Li, Z.; Fang, Z.; Zhang, Y.; Han, C. Ginsenoside Rb2: A Review of Pharmacokinetics and Pharmacological Effects. J. Ginseng Res. 2022, 46, 206–213.
- Jin, Y.; Hao, Y.; Zhang, H.; Qu, Z.; Wang, Y.; Piao, X. Dynamic Changes of Ginsenosides in Panax Quinquefolium Fruit at Different Development Stages Measured Using UHPLC-Orbitrap MS. Rapid Commun. Mass Spectrom. 2022, 36, e9270.
- Li, W.; Duan, Y.; Yan, X.; Liu, X.; Fan, M.; Wang, Z. A Mini-Review on Pharmacological Effects of Ginsenoside Rb3, a Marked Saponin from Panax genus. Biocell 2022, 46, 1417–1423.
- Bae, E.-A.; Choo, M.-K.; Park, E.-K.; Park, S.-Y.; Shin, H.-Y.; Kim, D.-H. Metabolism of Ginsenoside Rc by Human Intestinal Bacteria and Its Related Antiallergic Activity. Biol. Pharm. Bull. 2002, 25, 743–747.
- Kim, D.H.; Park, C.H.; Park, D.; Choi, Y.J.; Park, M.H.; Chung, K.W.; Kim, S.R.; Lee, J.S.; Chung, H.Y. Ginsenoside Rc Modulates Akt/FoxO1 Pathways and Suppresses Oxidative Stress. Arch. Pharm. Res. 2014, 37, 813–820.
- Yu, T.; Yang, Y.; Kwak, Y.-S.; Song, G.G.; Kim, M.-Y.; Rhee, M.H.; Cho, J.Y. Ginsenoside Rc from Panax Ginseng Exerts Anti-Inflammatory Activity by Targeting TANK-Binding Kinase 1/Interferon Regulatory Factor-3 and P38/ATF-2. J. Ginseng Res. 2017, 41, 127–133.
- Huang, Q.; Su, H.; Qi, B.; Wang, Y.; Yan, K.; Wang, X.; Li, X.; Zhao, D. A SIRT1 Activator, Ginsenoside Rc, Promotes Energy Metabolism in Cardiomyocytes and Neurons. J. Am. Chem. Soc. 2021, 143, 1416–1427.
- Chen, Y.-Y.; Liu, Q.-P.; An, P.; Jia, M.; Luan, X.; Tang, J.-Y.; Zhang, H. Ginsenoside Rd: A Promising Natural Neuroprotective Agent. Phytomedicine 2022, 95, 153883.
- Li, Y.; Wang, M.-L.; Zhang, B.; Fan, X.-X.; Tang, Q.; Yu, X.; Li, L.-N.; Fan, A.-R.; Chang, H.-S.; Zhang, L.-Z. Antidepressant-Like Effect and Mechanism of Ginsenoside Rd on Rodent Models of Depression. Drug Des. Devel. Ther. 2022, 16, 843–861.
- Peng, L.; Sun, S.; Xie, L.-H.; Wicks, S.M.; Xie, J.-T. Ginsenoside Re: Pharmacological Effects on Cardiovascular System. Cardiovasc. Ther. 2012, 30, e183–e188.
- Madhi, I.; Kim, J.-H.; Shin, J.E.; Kim, Y. Ginsenoside Re Exhibits Neuroprotective Effects by Inhibiting Neuroinflammation via CAMK/MAPK/NF-κB Signaling in Microglia. Mol. Med. Rep. 2021, 24, 698.
- Wang, H.; Lv, J.; Jiang, N.; Huang, H.; Wang, Q.; Liu, X. Ginsenoside Re Protects against Chronic Restraint Stress-Induced Cognitive Deficits through Regulation of NLRP3 and Nrf2 Pathways in Mice. Phytother. Res. 2021, 35, 2523–2535.
- Lee, B.; Shim, I.; Lee, H.; Hahm, D.-H. Effect of Ginsenoside Re on Depression- and Anxiety-like Behaviors and Cognition Memory Deficit Induced by Repeated Immobilization in Rats. J. Microbiol. Biotechnol. 2012, 22, 708–720.
- He, F.; Yu, C.; Liu, T.; Jia, H. Ginsenoside Rg1 as an Effective Regulator of Mesenchymal Stem Cells. Front. Pharmacol. 2019, 10, 1565.
- He, F.; Yao, G. Ginsenoside Rg1 as a Potential Regulator of Hematopoietic Stem/Progenitor Cells. Stem Cells Int. 2021, 2021, 4633270.
- Gao, Y.; Li, J.; Wang, J.; Li, X.; Li, J.; Chu, S.; Li, L.; Chen, N.; Zhang, L. Ginsenoside Rg1 Prevent and Treat Inflammatory Diseases: A Review. Int. Immunopharmacol. 2020, 87, 106805.
- Jiang, B.; Xiong, Z.; Yang, J.; Wang, W.; Wang, Y.; Hu, Z.-L.; Wang, F.; Chen, J.-G. Antidepressant-like Effects of Ginsenoside Rg1 Are Due to Activation of the BDNF Signalling Pathway and Neurogenesis in the Hippocampus. Br. J. Pharmacol. 2012, 166, 1872–1887.
- Li, X.; Xiang, N.; Wang, Z. Ginsenoside Rg2 Attenuates Myocardial Fibrosis and Improves Cardiac Function after Myocardial Infarction via AKT Signaling Pathway. Biosci. Biotechnol. Biochem. 2020, 84, 2199–2206.
- Liu, G.; Qi, X.; Li, X.; Sun, F. Ginsenoside Rg2 Protects Cardiomyocytes against Trastuzumab-Induced Toxicity by Inducing Autophagy. Exp. Ther. Med. 2021, 21, 473.
- Wang, Q.; Fu, W.; Yu, X.; Xu, H.; Sui, D.; Wang, Y. Ginsenoside Rg2 Alleviates Myocardial Fibrosis by Regulating TGF-Β1/Smad Signalling Pathway. Pharm. Biol. 2021, 59, 106–113.
- Gou, D.; Pei, X.; Wang, J.; Wang, Y.; Hu, C.; Song, C.; Cui, S.; Zhou, Y. Antiarrhythmic Effects of Ginsenoside Rg2 on Calcium Chloride-Induced Arrhythmias without Oral Toxicity. J. Ginseng Res. 2020, 44, 717–724.
- Cui, J.; Shan, R.; Cao, Y.; Zhou, Y.; Liu, C.; Fan, Y. Protective Effects of Ginsenoside Rg2 against Memory Impairment and Neuronal Death Induced by Aβ25-35 in Rats. J. Ethnopharmacol. 2021, 266, 113466.
- Wang, C.-Z.; Zhang, B.; Song, W.-X.; Wang, A.; Ni, M.; Luo, X.; Aung, H.H.; Xie, J.-T.; Tong, R.; He, T.-C.; et al. Steamed American Ginseng Berry: Ginsenoside Analyses and Anticancer Activities. J. Agric. Food Chem. 2006, 54, 9936–9942.
- Peng, Z.; Wu, W.W.; Yi, P. The Efficacy of Ginsenoside Rg3 Combined with First-Line Chemotherapy in the Treatment of Advanced Non-Small Cell Lung Cancer in China: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Front. Pharmacol. 2020, 11, 630825.
- Nakhjavani, M.; Smith, E.; Townsend, A.R.; Price, T.J.; Hardingham, J.E. Anti-Angiogenic Properties of Ginsenoside Rg3. Molecules 2020, 25, 4905.
- Kim, J.; Shim, J.; Lee, S.; Cho, W.-H.; Hong, E.; Lee, J.H.; Han, J.-S.; Lee, H.J.; Lee, K.W. Rg3-Enriched Ginseng Extract Ameliorates Scopolamine-Induced Learning Deficits in Mice. BMC Complement. Altern. Med. 2016, 16, 66.
- Tam, D.N.H.; Truong, D.H.; Nguyen, T.T.H.; Quynh, L.N.; Tran, L.; Nguyen, H.D.; Shamandy, B.E.; Le, T.M.H.; Tran, D.K.; Sayed, D.; et al. Ginsenoside Rh1: A Systematic Review of Its Pharmacological Properties. Planta Med. 2018, 84, 139–152.
- Zhang, H.; Park, S.; Huang, H.; Kim, E.; Yi, J.; Choi, S.-K.; Ryoo, Z.; Kim, M. Anticancer Effects and Potential Mechanisms of Ginsenoside Rh2 in Various Cancer Types (Review). Oncol. Rep. 2021, 45, 33.
- Lee, D.-Y.; Park, C.W.; Lee, S.J.; Park, H.-R.; Kim, S.H.; Son, S.-U.; Park, J.; Shin, K.-S. Anti-Cancer Effects of Panax Ginseng Berry Polysaccharides via Activation of Immune-Related Cells. Front. Pharmacol. 2019, 10, 1411.
- Wang, C.-Z.; Hou, L.; Wan, J.-Y.; Yao, H.; Yuan, J.; Zeng, J.; Park, C.W.; Kim, S.H.; Seo, D.B.; Shin, K.-S.; et al. Ginseng Berry Polysaccharides on Inflammation-Associated Colon Cancer: Inhibiting T-Cell Differentiation, Promoting Apoptosis, and Enhancing the Effects of 5-Fluorouracil. J. Ginseng Res. 2020, 44, 282–290.
- Xie, J.-T.; Wang, C.-Z.; Zhang, B.; Mehendale, S.R.; Li, X.-L.; Sun, S.; Han, A.H.; Du, W.; He, T.-C.; Yuan, C.-S. In Vitro and in Vivo Anticancer Effects of American Ginseng Berry: Exploring Representative Compounds. Biol. Pharm. Bull. 2009, 32, 1552–1558.
- Xie, J.T.; Wang, C.Z.; Ni, M.; Wu, J.A.; Mehendale, S.R.; Aung, H.H.; Foo, A.; Yuan, C.S. American Ginseng Berry Juice Intake Reduces Blood Glucose and Body Weight in Ob/Ob Mice. J. Food Sci. 2007, 72, S590–S594.
- Ko, S.K.; Bae, H.M.; Cho, O.S.; Im, B.O.; Chung, S.H.; Lee, B.Y. Analysis of Ginsenoside Composition of Ginseng Berry and Seed. Food Sci. Biotechnol. 2008, 17, 1379–1382.
- Hou, W.; Wang, Y.; Zheng, P.; Cui, R. Effects of Ginseng on Neurological Disorders. Front. Cell. Neurosci. 2020, 14, 55.
- Jin, Y.; Cui, R.; Zhao, L.; Fan, J.; Li, B. Mechanisms of Panax Ginseng Action as an Antidepressant. Cell Prolif. 2019, 52, e12696.
- Zhang, H.; Li, Z.; Zhou, Z.; Yang, H.; Zhong, Z.; Lou, C. Antidepressant-like Effects of Ginsenosides: A Comparison of Ginsenoside Rb3 and Its Four Deglycosylated Derivatives, Rg3, Rh2, Compound K, and 20(S)-Protopanaxadiol in Mice Models of Despair. Pharmacol. Biochem. Behav. 2016, 140, 17–26.
- Liu, M.; Liu, J.; Zhang, L.; Geng, Q.; Ge, Y. Antidepressant-like Effects of Ginseng Fruit Saponin in Myocardial Infarction Mice. Biomed. Pharmacother. 2019, 115, 108900.
- He, D.-F.; Ren, Y.-P.; Liu, M.-Y. Effects of Ginseng Fruit Saponins on Serotonin System in Sprague-Dawley Rats with Myocardial Infarction, Depression, and Myocardial Infarction Complicated with Depression. Chin. Med. J. 2016, 129, 2913–2919.
- Liu, M.-Y.; Ren, Y.-P.; Zhang, L.-J.; Ding, J.Y. Pretreatment with Ginseng Fruit Saponins Affects Serotonin Expression in an Experimental Comorbidity Model of Myocardial Infarction and Depression. Aging Dis. 2016, 7, 680–686.
- Hu, J.R.; Chun, Y.S.; Kim, J.K.; Cho, I.J.; Ku, S.K. Ginseng Berry Aqueous Extract Prevents Scopolamine-Induced Memory Impairment in Mice. Exp. Ther. Med. 2019, 18, 4388–4396.
- Shen, H.; Zhang, Z.; Jiang, S.; Jiang, Z. Protective effects of ginsenoside RB3 on hypoxic/ischemic brain injury and involved mechanisms. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2006, 22, 302–306.
- Wang, T.; Yu, X.; Qu, S.; Xu, H.; Han, B.; Sui, D. Effect of Ginsenoside Rb3 on Myocardial Injury and Heart Function Impairment Induced by Isoproterenol in Rats. Eur. J. Pharmacol. 2010, 636, 121–125.
- Sun, J.; Yu, X.; Huangpu, H.; Yao, F. Ginsenoside Rb3 Protects Cardiomyocytes against Hypoxia/Reoxygenation Injury via Activating the Antioxidation Signaling Pathway of PERK/Nrf2/HMOX1. Biomed. Pharmacother. 2019, 109, 254–261.
- Bansal, Y.; Singh, R.; Parhar, I.; Kuhad, A.; Soga, T. Quinolinic Acid and Nuclear Factor Erythroid 2-Related Factor 2 in Depression: Role in Neuroprogression. Front. Pharmacol. 2019, 10, 452.
- Jiang, S.; Fang, D.-F.; Chen, Y. Involvement of N-Methyl-D-Aspartic Acid Receptor and DL-α-Amino-3-Hydroxy-5- Methyl-4-Isoxazole Propionic Acid Receptor in Ginsenosides Rb1 and Rb3 against Oxygen-Glucose Deprivation-Induced Injury in Hippocampal Slices from Rat. Pharmacology 2018, 101, 133–139.
- Peng, L.-L.; Shen, H.-M.; Jiang, Z.-L.; Li, X.; Wang, G.-H.; Zhang, Y.-F.; Ke, K.-F. Inhibition of NMDA Receptors Underlies the Neuroprotective Effect of Ginsenoside Rb3. Am. J. Chin. Med. 2009, 37, 759–770.
- Jiang, S.; Miao, B.; Song, X.; Jiang, Z. Inactivation of GABA(A) Receptor Reduces Ginsenoside Rb3 Neuroprotection in Mouse Hippocampal Slices after Oxygen-Glucose Deprivation. J. Ethnopharmacol. 2011, 133, 914–916.
- Cui, J.; Jiang, L.; Xiang, H. Ginsenoside Rb3 Exerts Antidepressant-like Effects in Several Animal Models. J. Psychopharmacol. 2012, 26, 697–713.
- Kim, M.S.; Yu, J.M.; Kim, H.J.; Kim, H.B.; Kim, S.T.; Jang, S.K.; Choi, Y.W.; Lee, D.I.; Joo, S.S. Ginsenoside Re and Rd Enhance the Expression of Cholinergic Markers and Neuronal Differentiation in Neuro-2a Cells. Biol. Pharm. Bull. 2014, 37, 826–833.
- Kim, D.H.; Kim, D.W.; Jung, B.H.; Lee, J.H.; Lee, H.; Hwang, G.S.; Kang, K.S.; Lee, J.W. Ginsenoside Rb2 Suppresses the Glutamate-Mediated Oxidative Stress and Neuronal Cell Death in HT22 Cells. J. Ginseng Res. 2019, 43, 326–334.
- Han, S.-K.; Joo, M.-K.; Kim, J.-K.; Jeung, W.; Kang, H.; Kim, D.-H. Bifidobacteria-Fermented Red Ginseng and Its Constituents Ginsenoside Rd and Protopanaxatriol Alleviate Anxiety/Depression in Mice by the Amelioration of Gut Dysbiosis. Nutrients 2020, 12, 901.
- Liu, G.-M.; Lu, T.-C.; Sun, M.-L.; Jia, W.-Y.; Ji, X.; Luo, Y.-G. Ginsenoside Rd Inhibits Glioblastoma Cell Proliferation by Up-Regulating the Expression of MiR-144-5p. Biol. Pharm. Bull. 2020, 43, 1534–1541.
- Kim, C.; Spencer, B.; Rockenstein, E.; Yamakado, H.; Mante, M.; Adame, A.; Fields, J.A.; Masliah, D.; Iba, M.; Lee, H.-J.; et al. Immunotherapy Targeting Toll-like Receptor 2 Alleviates Neurodegeneration in Models of Synucleinopathy by Modulating α-Synuclein Transmission and Neuroinflammation. Mol. Neurodegener. 2018, 13, 43.
- Wang, X.; Sundquist, K.; Hedelius, A.; Palmér, K.; Memon, A.A.; Sundquist, J. Circulating MicroRNA-144-5p Is Associated with Depressive Disorders. Clin. Epigenetics 2015, 7, 69.
- Sundquist, K.; Memon, A.A.; Palmér, K.; Sundquist, J.; Wang, X. Inflammatory Proteins and MiRNA-144-5p in Patients with Depression, Anxiety, or Stress- and Adjustment Disorders after Psychological Treatment. Cytokine 2021, 146, 155646.
- Hyun, T.K. A Recent Overview on Ginsenosides as MicroRNA Modulators in the Treatment of Human Diseases. EXCLI J. 2021, 20, 1453–1457.
This entry is offline, you can click here to edit this entry!
