Bacteriophages (phages for short) are viruses, which have bacteria as hosts. The single phage body virion, is a colloidal particle, often possessing a dipole moment. As such, phages were used as perfectly monodisperse systems to study various physicochemical phenomena (e.g., transport or sedimentation in complex fluids), or in the material science (e.g., as scaffolds). Nevertheless, phages also execute the life cycle to multiply and produce progeny virions. Upon completion of the life cycle of phages, the host cells are usually destroyed. Natural abilities to bind to and kill bacteria were a starting point for utilizing phages in phage therapies (i.e., medical treatments that use phages to fight bacterial infections) and for bacteria detection. Numerous applications of phages became possible thanks to phage display—a method connecting the phenotype and genotype, which allows for selecting specific peptides or proteins with affinity to a given target.
- bacteriophages
- phage display
- phage therapies
- phage-based sensors
- soft matter
- materials
- scaffolds
- viruses
- Introduction
Nanoscience is “enabling technology”, which impacts numerous fields of research and everyday life. The socio-economic implications of introducing new nanotechnology or a class of nanomaterials can be, therefore, far-reaching (across many economic sectors) and very deep (starting at the beginning of the value/supply chain) [1]. The most widely used in the industry is the “top-down” approach for the preparation of nano-objects. “Top-down” utilizes external tools and forces the creation of smaller objects from larger entities (via e.g., milling, lithography, printing). It seems that the developments in the field are reaching their limit, with Intel not being able to master a 7 nm process for many years now [2]. Alternatively, in the “bottom-up” approach, individual parts self-assemble spontaneously to form ordered and/or functional structures due to specific interactions, often imposed on these building blocks. Despite all the advances that nanotechnology has brought to us, the abiotic self-assembling systems are relatively simple, and their functionalities are restricted. Even with the introduction of dynamic self-assembly, which allowed to design systems consuming energy to maintain their structure and functions [3], and some advantages in creating chemical networks [4], we are still far behind the level of complexity present in nature. Nevertheless, we have learned to take advantage of biotic functional systems and incorporate some “natural” building blocks into human-made nanodesigns. Here, we review the specific manifestation of such an approach, namely the utilization of bacteriophages (phages for short), i.e., viruses, which have bacteria as hosts.
The average size of the virion, i.e., a single phage body, is around 30–200 nm, but the largest might be more than 800 nm in length [5]. The most popular phages fit perfectly into the category of nano-objects, i.e., having at least one geometrical dimension smaller than 100 nm. Nature offers a great variety of the possible structural morphologies of phages. The majority belong to the order of Caudovirales and share a typical structure design, i.e., the genetic information is stored in a capsid, to which a spike-tail with fibers is attached [6,7]. Less common are filamentous (e.g., M13) or nearly spherical (isometric) phages (e.g., MS2). Filamentous phages are broadly used, as they are most robust in phage display (cf. Section 2.1), and they form liquid crystalline phases (cf. Section 4). MS2 is used as a model of eukaryotic viruses in a number of bio-related studies [8]. T1, T4, T7, or Lambda phages are the most well known examples of tailed phages and are often chosen as model systems representing the most abundant Caudovirales order. It is estimated that there are around 1031 phages on the planet, making them the most abundant and diverse biological entities. They have a profound role as modulators in numerous biomes, ranging from the human gut to biogeochemical cycling in aquatic environments [7].
Phages can be produced easily and cheaply in large quantities and easily purified. By only infecting a bacteria solution, one can obtain a large number of progeny phages. Phages undergo evolution, and thus they remain effective against bacteria [9]. For example, anti-CRISPR (Anti-Clustered Regularly Interspaced Short Palindromic Repeats) [10] mechanism was discovered shortly after CRISPR [11]—a molecular mechanism that allows bacteria to recognize and degrade alien genetic material. Some phages are robust and retain their activity even after exposure to high temperatures [12], pH [13], and organic solvents [14,15]. Each virion of a given phage is identical (no polydispersity of sizes), whereas chemical and genetic modifications allow for the preparation of the virions of desired properties. All these traits make phages exciting building blocks for utilization in nanotechnology.
- Bacteriophages in Bio-Related Applications
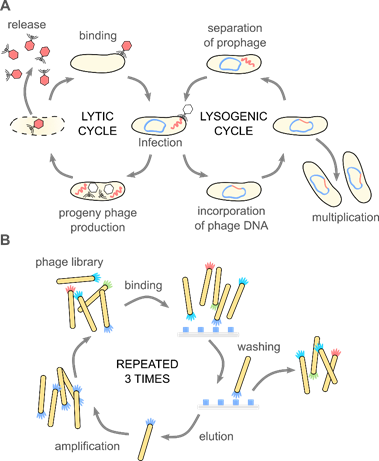
Phages attach to the host cell and introduce genetic materials inside bacteria. What happens next determines the qualification of bacteriophages as a lysogenic and lytic one (see Figure 1A). In the lytic cycle, a host’s cell is disrupted to free progeny phages from the infected bacteria. Lysis is possible due to the amurins, which are proteins that inhibit peptidoglycan synthesis [16]. In the lysogenic cycle, the viral genome integrates into the chromosome of bacteria and remains latent, replicating for generations [17]. When viral genetic material is incorporated into the chromosomal DNA of bacteria, it is known as a prophage [18]. The appearance of stressors, e.g., chemicals, UV radiation, or damage of the host DNA, can cause the conversion of the cycle and change from lysogenic into the lytic [19]. Only some filamentous phages might cause the continuous generation of progeny virions without causing the death of the host [20]. Bacteriophages are considered non-toxic to eukaryotes because structural elements of the virion cannot bind to eukaryotic cells [21].
Figure 1. (A) Bacteriophages are obligate parasites, which usurp the molecular machinery of the host to complete their life cycle. First, phage virions bind to target bacteria and inject the genetic information into the host cell. Only the recognition of a proper host assures the completion of the cycle. In the case of lytic phages, progeny virions are produced inside the host cell. The release of the progeny virions usually results in the disruption and death of the bacterium. Temperate phages integrate their genetic information with the genome of the host, forming a prophage. A prophage replicates upon divisions of the bacteria. External stimuli (e.g., food shortage, UV light, temperature) might activate the prophage, and thus start the lytic cycle. These natural mechanisms (i.e., binding to specific target bacteria and amplification), along with the monodispersity of virions and a variety of morphologies, are fundamental for the application of phages in phage therapies, biocontrol, sensing, material science, and soft matter research. (B) The discovery of the phage display method significantly broadened the possible usage of bacteriophage. In the phage display, a fusion of large libraries with genes coding coat proteins allows for a direct correlation between the genome and phenotype. Bio-panning allows for selecting specific peptides or proteins with affinity to a given target. These selected peptides might be used separately or provide phage virions with additional functionalities. Phage display was crucial in the development of new drugs, vaccines, but also material science and physical chemistry.
2.1. Phage Display and Phage-Based Delivery Systems
One half of the 2018 Nobel Prize in Chemistry was awarded to George P. Smith and Sir Gregory P. Winter “for the phage display of peptides and antibodies” [22]. The method utilizes the possibility to genetically modify phages to display peptides, proteins, or antibodies at the surface via fusion with appropriate gene product. This results in a connection between genotype and phenotype. The exposition of phages possessing various inserts to a target material allows for the screening of large libraries in search of peptides or even proteins that interact with the target. Bio-panning allows for selecting specific peptides or proteins with affinity to a given target (see Figure 1B). Biopanning involves four major steps: (1) the preparation of phage display libraries, (2) capturing steps, where virions displaying sequence having affinity to the target bind to it, (3) washing step, which removes unbound virions, and (4) the elution step, which allows collecting phages with specific affinity. In the earliest examples, genes were cloned directly into the phage genome. Alternatively, phagemid vectors are used; in such a case, “helper phage” is needed to produce functional virions. The M13 phage, and the closely related fd and f1, are the most extensively used in phage display [23].
The possibility to probe and identify ligand–receptor interactions allowed for advancement in studies of infectious diseases [24] and cancer [25,26]. This resulted in improvements in drug discovery and vaccine design [27]. The overview of the phage-display method and its utilization in medicine can be found in recent reviews by Mimmi et al. [28], Sokullu et al. [29], Petrenko [30], Garg [31], Sunderland et al. [32], and Newman and Benoit [33].
In a new example by Lauster and coworkers [34], the modification of a phage capsid with non-natural amino acid (L-homopropargylglycine) was introduced to the coat protein for spatial control over the distribution of ligands binding to the spike protein of the influenza A virus. The appropriate geometrical distribution of ligands was possible due to the symmetric, icosahedral structure of bacteriophages Qβ. These capsids bind to the influenza A virus envelope in the multivalent mode, thus inhibiting the infection of eukaryotic cells.
Not only medicine but also nanotechnology are taking advantage of the phage-display technique. There are examples of phage display-selected peptides for the binding of nanoparticles (e.g., noble metals [35], ZnO [36], Fe3O4 [37,38], semiconductors TiO2, CdS, ZnS, (SiO2) [39–43]), minerals [44] or ions (e.g., arsenic (III) [45]). The potential applications vary from sensing [45–47], separation, and processing [44] to templated nanoparticle synthesis, where phage display-selected peptides control the nucleation and growth of inorganic nanoparticles [48,49].
Bacteriophages are also utilized as delivery carriers [50]. Displaying specific peptides targeting eukaryotic cells or tissues compensates for the lack of natural mechanisms to enter mammalian cells or reach appropriate intracellular compartments. Non-modified phages are sometimes used, but the efficiency of virions containing cell-specific targeting peptides developed by phage display [51] or even phage-inspired nano-carriers [52] is superior. There is a possibility of coupling a variety of loads (e.g., drugs) by genetic manipulation [53,54] or chemical conjugation [55,56]. Upon the recognition of the target, payloads might be released in a controlled manner. Hyman said that even despite the size of phages, they “only very loosely fit into the nanotechnology category” [57]. Therefore, here we focused on chemically modified systems and artificial designs.
Lambda phage was used as early as 1971 to deliver the gene for galactose transferase into human fibroblast cells isolated from a patient with a deficiency in this enzyme [58]. M13 was used for the targeted delivery of the GFP gene-expression cassette into breast cancer cells [59]. Filamentous phage f1 with targeting antibodies displayed on the tip of the virion was used as a carrier of chemically conjugated antibiotics [60]. Authors showed that such nanomedicine is non-toxic to mice, its immunogenicity is reduced in comparison to native phages, whereas the half-life of the conjugated phage increased in the bloodstream. Icosahedral MS2 phage might undergo genetic manipulation, chemical conjugation, and the removal of genetic material resulting in an empty nano-carrier, which might be loaded with the cargo of choice. For instance, Stephanopoulos et al. [61] showed the dual modified virus capsids: the cell-specific aptamer was conjugated to the unnatural amino acids (p-aminophenylalanine) displayed at the outer surface, whereas porphyrins were attached to the specially introduced cysteine residues at the inner surface. Upon the illumination of porphyrins, singlet oxygen was generated, which led to the selective destruction of target cells.
Recently, Zhu et al. [62] showed a prokaryotic–eukaryotic hybrid, composed of T4 phage and adeno-associated virus (AAV). The bacteriophage acted as the cargo, which might be reasonably easily modified. Th eukaryotic virus acted as a “driver” that allowed for cargo delivery into mammalian cells. The authors showed the example of the hybrid vector, where double stranded luciferase plasmid was packed into a T4 capsid, β-galactosidase was displayed at the phage capsid, and additionally, single stranded GFP DNA was packed in AAV capsid. This load was delivered simultaneously into the cell. The luciferase activity was four orders of magnitude larger compared to only T4 virions.
Phage proteins themselves can also assemble into nano-carriers for gene delivery [52]. For instance, the Petrenko group showed the encapsulation of siRNA by fused protein composed of phage capsid protein and targeting moiety [63]. The phage-mimetic nanoparticle was named “nanophage” as it comprises both protein shell and genetic material stored inside. “Nanophages” were used to deliver siRNA to the target region and cause gene silencing.
Phage-displayed peptides were also used to create artificial, nanoparticulate delivery systems. For instance, chitosan nanoparticles were conjugates with a phage-display selected sequence targeting the follicle-associated epithelium region of Peyer’s patch [64]. This work is an example of a potential carrier for vaccine delivery. Other polymer-based nanoparticles, namely polyethylene glycol–poly(lactic-co-glycolic acid) (PEG–PLGA), modified with phage-displayed peptides, were used to target the brain [65]. The possibility to deliver drugs through the blood–brain barrier might impact the therapy of many central nervous system diseases. Interestingly, Ma et al. [66] showed the utilization of liposome modified with a specific phage-displayed peptide and loaded with DNA to target cell nuclei. The authors delivered the transposon system, which allowed the efficient insertion of transgenes into the host genome, into the nuclei of rat mesenchymal stem cells. Such a non-viral gene delivery vector is promising for stem cell therapy. Jin et al. [67] found a series of peptides prolonging the blood residence time for M13 bacteriophage. Later, they transferred this property to self-assembled heavy-chain ferritin nanocages. In addition, metal nanoparticles were decorated with specific, phage-displayed peptides. For instance, Wang et al. [68] developed Au@Ag heterogenous nanorods, which were directed by fusion proteins into cancer cells, where metallic nanostructures were used for photothermal ablation. In a similar example, Peng et al. [69] conjugated gold nanorods with chimeric phages that were engineered to specifically attach to several Gram-negative bacteria. Upon excitation by near-infrared light, gold nanorods release energy locally, generating heat that efficiently kills targeted cells.
In a different approach, bacteriophages were used as a molecular label [70]. The information was included in the DNA, which was later read using PCR-driven amplification. Bacteriophages were applied to label a nanoscaled bulk material, e.g., multi-walled carbon nanotubes.
2.2. Phages as Antibacterial Agents: Phage Therapy, Biocontrol Applications
The intrinsic efficiency of phages against bacteria resulted in the development of phage-derived antibacterials [71]. Bacteriophages have been used for medical purposes since the early 1900s [17,72]. Phages can support the development of inflammatory response against bacteria via the lysis of bacterial cell wall, which activates the immune system [73]. Therefore, phage therapies, i.e., the administration of phage cocktails to patients infected with bacteria, besides the direct elimination of bacteria cells, additionally activate the human immune system to fight against infection. Phage therapies were largely abandoned when antibiotics were developed [74]. However, the spread of drug-resistant superbugs and the lack of new medicines [75,76] has caused a renaissance of bacteriophage-based antimicrobials [77]. Phages are therapeutically used to treat bacterial infections that do not respond to conventional antibiotics, particularly in Russia and Georgia [78]. In these countries, phage products are directly available, even without a prescription (e.g., “Intestiphage”) [79]. The Russian company Microgen sells phages as liquid preparations or as pills [80]. In recent years, phage-based treatments reached clinical trials, e.g., curing inner ear infections [81], typhoid [82], or infected burn wounds (“Phagoburn” project) [83]. The “Phagoburn” project was terminated early because the utilized phage cocktails were proved to not be effective [83]. In 2019, the Food and Drug Administration approved the first clinical trial in the USA for intravenous phage therapy [84]. The very compelling reviews on the history of phage therapies are given by Abedon et al. [79] and Cisek et al. [16]. The summary of the current situation is given by Altamirano and Barr [85].
The ability of phages to kill target bacteria is also used in biocontrol applications, e.g., in the food industry and agriculture. The application of phages is safer compared to the utilization of antibiotics [86]. In 2017, the European Commission adopted the “EU One Health action plan against antimicrobial resistance”. It was the first step toward the control of the use of antibiotics in agro-food production, as it is a major source of bacterial resistance acquisition [87]. Phages were used to protect dairy products [88], fruits [89], vegetables [90], meat [91], and fish [92]. Developments in the utilization of phages as antimicrobial agents in plant and animal agriculture at the farm level are summarized in a recent review by Svircev et al. [93].
This entry is adapted from the peer-reviewed paper 10.3390/nano10101944