Endometriosis is a chronic disease in which endometrial tissue grows outside the uterus. Endometriotic implants most commonly occurs in the pelvis, involving ovaries, peritoneum, uterosacral ligaments, rectovaginal septum and vesico-uterine fold, but less frequently they can appear in another site in the body such as umbilicus, diaphragm, bowel, pleura and pericardium. An estimated 5–10% of reproductive-age women, or approximately 176 million women worldwide, are affected by this disease. Although some women do not experience symptoms associated with endometriosis, the disease is more commonly responsible for painful symptoms (such as dysmenorrhea, non-menstrual pelvic pain, dyspareunia, dysuria, dyschezia) and infertility
[1]. Pain negatively impacts sexual activity, energy work, social life and overall quality of life (QoL), representing the most debilitating symptom in women with endometriosis. Many societies including the American Society for Reproductive Medicine (ASRM) and the American College of Obstetricians and Gynecologists (ACOG) suggest empiric treatment before definitive surgical diagnosis
[2][3]. In spite of surgical procedures for the treatment of endometriosis that significantly improve pain symptoms, they can be associated with complications
[4] and the pain relapse rate after surgery is not negligible
[5]. Furthermore, the risk of damage to the ovarian reserve must be considered when ovarian endometriomas are treated surgically
[6]. Accordingly, medical treatment plays a key role in long-time management of this disease
[7][8]. The ongoing hormonal therapies are not able to improve infertility associated with endometriosis, but they can only ameliorate painful symptoms
[7]. Endometriosis cannot be resolutely eliminated with hormonal therapy, as it persists and even progresses in spite of the effectiveness of drugs in improving symptoms
[9]. Indeed, pain generally recurs when patients discontinue therapy due to the onset of adverse effects or the desire for pregnancy. Hormone treatments available for symptomatic endometriosis work by suppressing ovulation and uterine blood flow, and by reducing serum estradiol levels, thus resulting in a estrogen-deficiency state
[9]. Combined oral contraceptives (COCs) and progestins are the drugs of choice for treating symptoms related to endometriosis. Careful diagnostic examination of women with endometriosis should be performed before opting for second-line hormonal therapies, including gonadotropin-releasing hormone analogs (GnRH-as) or aromatase inhibitors (AI). Furthermore, different new molecules have been studied in vitro and in animal models of disease since the knowledge of the molecular pathways underlying endometriosis’ pathogenesis has increased
[10]. Pain control is the main goal of endometriosis treatment because this is accompanied by an improvement in the QoL and the burden of disease. Moreover, drugs find their application in decreasing surgical interventions, improving postoperative pain control and even achieve disease remission. Since endometriosis is a benign but chronic disease it is of the utmost importance to select pharmacotherapies that maximize benefits and minimize side effects.
2. Endometriosis-Related Pain
The pelvis is highly innervated and vascularized, which allows pain impulses to be processed and sent from this region to the brain
[11]. This, along with many other factors, supports the painful syndrome associated with endometriosis. High levels of nerve growth factors that promote neurogenesis have been found in the peritoneal fluid of patients with endometriosis; the ratio of sympathetic to sensory nerve fibers is significantly altered within endometriotic lesions and the nerve density within endometriotic nodules is increased
[12][13]. Additionally, prostaglandins and cytokines released by inflammatory cells appealed to ectopic endometrial-like tissue can activate nerve fibers and nearby cells to produce inflammatory molecules
[11]. Entrapment of nerve fibers within endometriotic tissues also contributes to the genesis of pain
[11]. Cyclic sciatic pain, sensory loss, and weakness can result from endometriotic entrapment of the lumbosacral, femoral, and sciatic nerve roots. There are several cases of sacral radiculopathy occurring in patients with endometriosis and there are even descriptions of women in wheelchairs who become fully ambulatory after treatment of deep infiltrating endometriosis
[14]. Another mechanism that promotes endometriosis-related pain is the central sensitization. Women become highly sensitive to subsequent painful stimuli due to endometriosis-induced neuroplastic changes in the descending pathways that modulate pain perception. In response to a subsequent insult (i.e., nephrolithiasis or pelvic organ injury), patients may experience endometriosis-like pain due to the inability to trigger descending pathways of inhibition
[15].
3. Estro-Progestins and Endometriosis
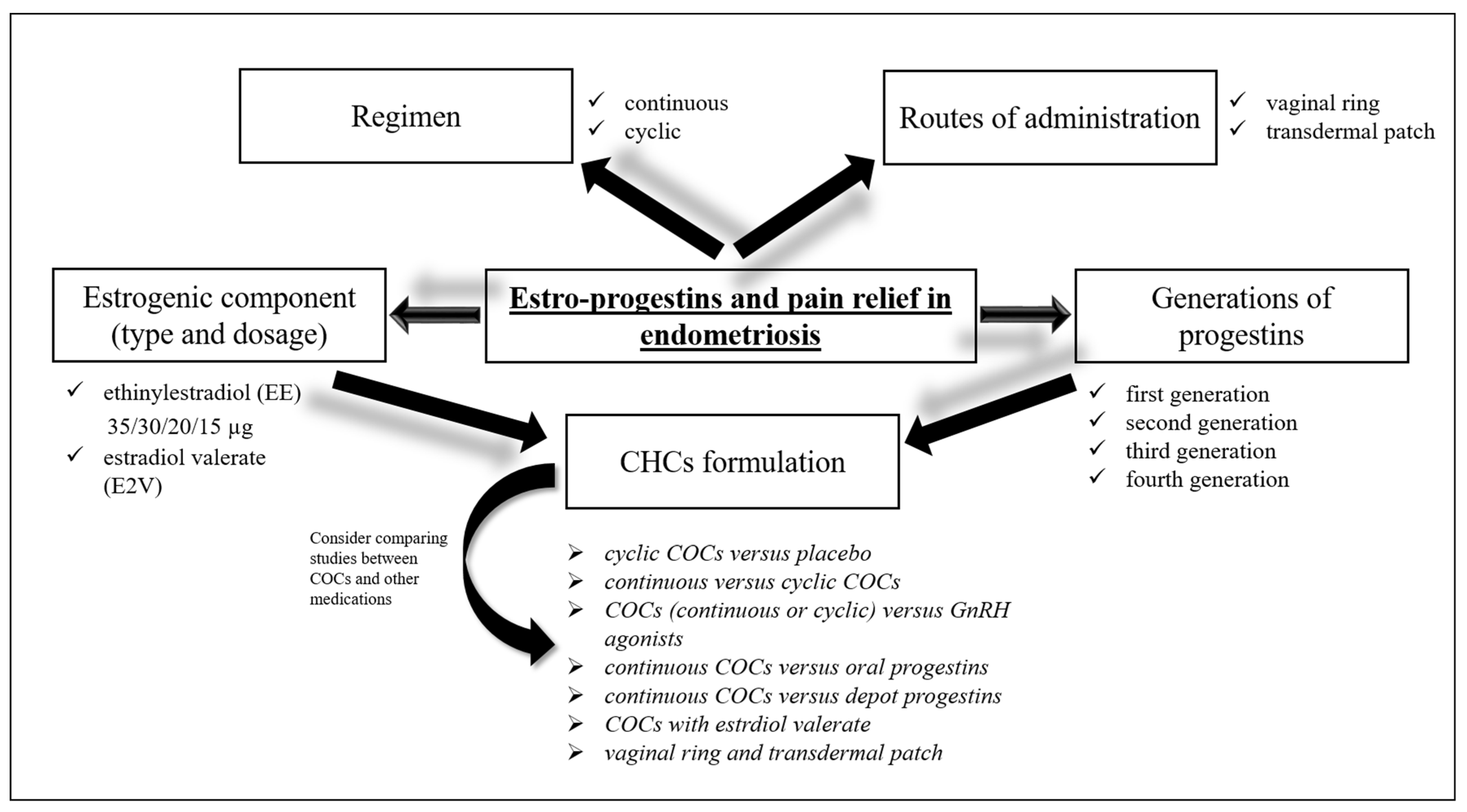
Since ovulation and menstruation play an important role in the pathogenesis of endometriosis, the therapeutic key for the control of the disease and associated symptoms would be hormonal treatment, leading to suppression of both conditions. Combined hormonal contraceptives (CHCs) are indeed an efficacious therapeutic option for the management of endometriosis-related symptoms in patients who also require effective contraception. Over the last 60 years COCs have undergone notable changes: starting from exclusive use of synthetic estrogen ethinylestradiol (EE) at progressively lower doses, to introduction of estradiol (E2)—valerate or micronized—a natural estrogen produced by granulosa cells of the ovaries
[16]. On the other hand, several generations of progestins were combined with the estrogenic component and different molecules of progestins have been tested with the aim of obtaining tailoring contraceptive options that could meet the different needs of patients. The introduction of alternative routes of administration to the oral one, such as intravaginal, transdermal, subdermal, intrauterine and injectable represented another considerable step in the technology of hormonal contraceptives (
Figure 1)
[16]. The guidelines of European Society of Human Reproduction and Embryology
[17] recommend treating women with hormonal contraceptives to improve endometriosis-related symptoms. However, there is no clear evidence on which specific preparation, among the numerous combinations of CHCs available, should be utilized based on endometriosis stage and type, and woman’s age to obtain a targeted treatment. When analyzing studies on estro-progestins for the treatment of symptoms related to endometriosis the results should be interpreted considering also the main methodological differences related to eligibility requirements, treatment assignment, and outcome assessments. Some studies required a surgical diagnosis of disease, others used radiological criteria or both methods. The study methods are manifold: there are randomized controlled trials (RCTs) (double-blind or open-label) or observational studies; among the latter some did not have a comparison group so they compared post-therapy scores with the baseline ones, while other observational studies used a comparative design. Furthermore, most observational comparative studies applied a patient preference design in which each participant could decide which treatment group to belong to. Visual analogue scale (VAS) or verbal rating scale (0–10 cm or 0–100 mm) based on Andersch and Milsom or Biberoglu and Behrman scales are the most frequently used pain assessment tools. A difference in pain score ≥10 mm on a 100 mm VAS may be considered clinically significant, although a greater difference is required if consistent differences emerge between the treatments compared
[18].
Figure 1. Estro-progestints in endometriosis. CHCs are an efficacious therapeutic option for the management of endometriosis-related symptoms. The different routes of administration (oral, vaginal, transdermal), the regimen (continuous versus cyclic), and the estrogenic and progestin component should be considered to obtain a targeted treatment. However, there is no clear evidence on which specific preparation, among the numerous combinations of COCs available, should be utilized based on endometriosis stage and type, and woman’s age. There is no strong evidence to establish the comprehensive superiority of COC therapy and its benefits over other approaches. A step-by-step approach based on the use of COCs as the first line, progestogens (including POPs) as the second step, and GnRH agonists and antagonists as the third step was recommended. CHCs: combined hormonal contraceptives. COCs: combined oral contraceptives.
Unlike the VAS scale, which allows for an accurate and well-validated measurement of endometriosis-associated pain, neither the Biberoglu and Behrman scales nor the Andersch and Milsom scales have been validated, leading to confusion and methodological limitations
[18]. Differences in clinical practice and in the characteristics of enrolled patient populations also represent study limitations. Others relevant concerns such as duration of therapy, COCs formulation and regimen (continuous or cyclic), and the route of administration should be considered when comparing studies (
Figure 1).
3.1. Cyclic COCs vs. Placebo
Harada et al. in two different studies used a double-blind placebo-controlled design to evaluate COCs effectiveness in Japanese patients suffering from pain associated with endometriosis. In the first study, one hundred patients with endometriosis-related dysmenorrhea were randomly assigned to receive four cycles of either monophasic COC (EE 35 µg plus norethisterone 1 mg) or placebo
[19]. Total dysmenorrhea scores assessed by a verbal rating scale significantly decreased at the end of treatment in both the COCs and placebo groups. Nevertheless, dysmenorrhea VAS scores reduction exceeded the minimum clinically significant threshold only among COCs users (31.1 mm), with three-fold greater reduction compared to the placebo group (difference of 9.6 mm). This statistically significant difference between groups was recorded from the first course of treatment and continued until the end of treatment. There was no clinically significant reduction in non-menstrual pain in COCs users
[19]. Endometriomas larger than 3 cm in diameter significantly reduced their volume in the COCs group, but not in the placebo group
[19]. Almost all of the patients (approximately 95%) had endometriomas diagnosed only by ultrasound in the absence of a surgical diagnosis, representing a study limitation. This could hide a higher percentage of more advanced disease in the population enrolled than is usually observed in clinical practice. Safety and efficacy of EE 20 µg plus drospirenone 3 mg were evaluated in another double-blind, placebo-controlled, parallel-group study by Harada and colleagues; an extended flexible regimen versus placebo for the treatment of pelvic pain associated with endometriosis was investigated
[20]. A total of 312 Japanese patients with endometriomas predominantly diagnosed by ultrasound were randomized to a flexible extended regimen, placebo, or dienogest. The extended flexible regimen and placebo arms took 1 tablet daily without interruption for 4 months, with a 4-day tablet-free interval after 4 months or after ≥3 consecutive days of spotting and/or bleeding on days 25–120. After 24 weeks, placebo recipients were changed to a flexible extended regimen. Patients randomized to dienogest received 2 mg/day for 52 weeks in an unblinded reference arm. Compared with placebo, a flexible extended regimen significantly reduced severe pelvic pain assessed using a 100-mm VAS (mean difference in pain score −26.3 mm). However in the open-label parallel group treated with dienogest the pain score decreased even more (decrease of 50.0 mm)
[20]. A flexible extended regimen also improved other endometriosis-associated pain and gynecologic findings and reduced endometriomas size. Pelvic pain improved after therapy despite no reduction in the number of bleeding/spotting days
[20].
3.2. Continuous vs. Cyclic COCs
Only one observational comparative trial conducted by Caruso et al. analyzed the effects of a continuous versus a 21-day cyclic regimen of EE 30 µg plus dienogest (DNG) 2 mg on sexuality and QoL in patients with pelvic pain (63 versus 33 patients). VAS measurements at 3 and 6 months revealed a significant improvement in endometriosis-related pain from baseline with the continuous regimen, while cyclic use resulted in a significant pain reduction at only 6 months. Despite the continuous regimen showing greater improvements than cyclical COC use, no statistically significant comparisons between groups were reported
[21].
3.3. COCs (Continuous or Cyclic) vs. GnRH Agonists and Antagonists
Continuous COCs treatment comparing with GnRH agonist plus hormonal add-back therapy was investigated by Guzick et al. in a randomized double-blind study
[22]. Forty-seven patients with endometriosis-related pelvic pain received a daily capsule containing COCs (EE 35 µg plus norethindrone 1 mg) or add-back norethindrone acetate 5 mg and an intramuscular injection of placebo or depot leuprolide 11.25 mg every 12 weeks. The verbal rating score, Biberoglu and Behrman scale, Beck Depression Inventory, and Index of Sexual Satisfaction were used to evaluate changes in pelvic pain over 48 weeks. Both treatment groups resulted in a significant improvement in pain from baseline and there was no significant difference in the extent of pain relief between the two arms. In both regimens improvements were evident from the first evaluation after 28 days
[22]. Given the lower cost and generally low side effects of COCs, these findings support the efficacy of continuous administration of COCs as first-line therapy in the medical management of symptomatic endometriosis. Other studies have instead compared cyclic COCs with GnRH agonists treatment. Vercellini et al. in a open-label, randomized trial evaluated the efficacy of six-month treatment with goserelin versus a low-dose cyclic COCs in improving pelvic pain in fifty-seven patients with moderate or severe pelvic pain with surgical diagnosis of endometriosis. A significant reduction in deep dyspareunia was recorded at 6 months of therapy in both groups, with goserelin being superior to COCs. Patients taking COCs experienced a significant improvement in dysmenorrhea and non-menstrual pain had decreased with no difference between groups. At the end of the follow-up, symptoms reappeared with no differences in severity between treatments
[23]. Zupi et al. randomized into three groups one hundred thirty-three women with pelvic pain recurrence after endometriosis surgery for 12 months: group 1 with leuprolide acetate (LA) alone, group 2 with LA plus add-back therapy (transdermal E2 and oral norethindrone), or group 3 with cyclic COC (EE 30 µg plus gestodene 0.75 mg). Groups 1 and 2 showed greater pain improvement compared to oral contraceptive therapy; in addition, patients treated with add-back therapy showed a reduced rate of adverse effects and better QoL than the other two treatments. Add-back therapy allows women with pain recurrence to be treated for a longer time, with decreased bone mineral density loss, good pain control, and better QoL compared with GnRH agonist alone or COCs
[24]. Parazzini et al. compared 12 months of COC use (EE 30 µg plus gestroden 0.75 mg) with 4 months of GnRH agonist therapy followed by 8 months of COC and no significant differences between groups in pain relief were found
[25]. The pilot study of Di Francesco and Pizzigallo evaluated the efficacy of treatment with micronized palmitoylethanolamide + trans-polydatin (a food supplement anti-inflammatory agent) in comparison to usual hormonal therapies
[26]. Thirty outpatients of reproductive age with a history of chronic pelvic pain associated to endometriosis were randomly assigned to three groups of 10, who underwent a 6-month treatment with: palmitoylethanolamide + trans-polydatin, leuprorelin acetate or cyclic COCs (EE 30 µg plus drospirenone 3 mg). Dysmenorrhea, chronic pelvic pain, and dyspareunia intensity significantly decreased over time in all three groups, irrespective of the treatment applied. In spite of the study’s limited sample size, the data demonstrate that palmitoylethanolamide + trans-polydatin is as effective as hormonal therapy in reducing painful symptomatology related to endometriosis in patients of reproductive age, without suppressing ovulation, allowing to conceive where possible and showing excellent tolerability
[26]. The lack of adequate blinding and an unclear randomization scheme represent the limitations of these studies.
Due to higher cost, limited accessibility, hypoestrogenic side effects GnRH agonist are usually considered as second-line therapy. Long-term GnRH agonist treatments leads to loss of bone density together with hypoestrogenic status that comes with alteration of lipid profile, hot flushes, urogenital atrophy, headaches and depression. For these reasons they should be used no longer than 6 months and an hormonal add-back therapy is strongly suggested.
Promising preliminary results are available for oral elagolix, a new gonadotropin-releasing hormone antagonist, which is under investigation in multicenter Phase III trials
[9][27]. Currently, two ongoing Phase III trials are evaluating the safety and efficacy of elagolix tablets in combination with combined oral contraceptive tablets to assess dysmenorrhea response in premenopausal women with endometriosis and associated moderate to severe pain (NCT03213457 and NCT04333576).