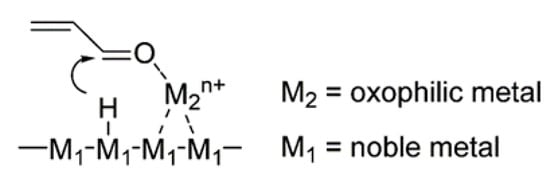Among the works on 5-hydroxymethylfurfural (HMF) hydrogenation reported in the literature, it can be seen that most of the catalysts used consist of a noble metal, such as Pd, Ru or Pt, apart from transition metal catalysts based on Cu, Ni and Co supported on metal oxides and carbon. The addition of a secondary metal from a transitional metal to a noble metal has also been used by many researchers. Apart from the different types of metal, it was also observed that reaction conditions, namely the type of solvent, H2 donor, reaction temperature, reaction time and H2 pressure, play an important role in influencing the HMF conversion and 2,5-dimethylfuran (DMF) yield.
Dumesic et al. were able to produce a reasonable DMF yield using CuCrO4 and CuRu/C catalysts under a batch condition in 1-butanol at 220 °C and 6.8 bar H2 in 10 h (entry 2). It was observed that the addition of Ru to Cu supported on carbon increased the DMF yield from 61% to 71% under the same reaction condition. Although the Ru/Co3O4 (entry 13)-catalysed reaction achieved a high DMF yield (93%) at relatively low pressure and temperature, a high catalyst loading (40 wt% to HMF) and long reaction time (24 h) are needed. Pd/C in supercritical carbon dioxide leads to a 100% yield of DMF in 2 h; however, the reaction requires a specific combination of water and supercritical CO2 [entry 12].
DMF production by catalytic transfer hydrogenation has also been investigated using formic acid (FA)
[1][2], methanol
[3] and isopropyl alcohol (IPA)
[4][5]. Thananatthanachon et al. demonstrated the conversion of HMF to DMF using Pd/C/H
2SO
4 (2 mmol HMF in THF, 0.4 g of catalyst) with 95% yield of DMF in 15 h at 120 °C
[6]. However, this requires the addition of H
2SO
4. Dutta et al. performed the same reaction using Ru/C; however, a lower DMF yield was obtained (30%)
[7].
2. Hydrogenation with Noble Metal Catalysts
Typical processes to acquire upgraded biofuels or chemical platforms require the use of homogeneous, heterogeneous or biological (enzymes, microorganisms and yeasts) catalysts
[8]. This process normally involves acid-catalysed reactions. As the conversion of HMF to DMF is a hydrogenation/hydrogenolysis process, catalysts used for this reaction are hydrogenation catalysts, commonly noble metals such as Pd
[9], Ru
[1][10][11], Pt
[12] and others, which are generally supported on carbon in some form. This combination provides high reactivity and good dispersion on sustainable and tuneable supports with a high surface area and easy-to-modify porosity and surface properties.
However, noble metals may be deactivated in water, which is commonly used in these hydrogenation reactions. Moreover, their price is high and unsustainable. Only in the case of low concentrations and high catalyst lifetime can these catalysts be considered as those of the future for biomass transformations. However, they possess high reactivity even at low hydrogen pressures, and it was shown that it is possible to obtain 100% HMF conversion and 98% DMF yield after 2 h at 180 °C and 10 bar H
2 over Pt-Co in hollow carbon nanospheres
[12]. In another study, Ru/Co
3O
4 catalyst exhibited 93.4% DMF yield at 130 °C and 7 bar H
2 [11]. Further still, transfer hydrogenation with 2-propanol as a hydrogen donor was carried out by Jae and co-workers, who used Ru/C and achieved 100% HMF conversion and 81% DMF selectivity after 6 h at 190 °C under 20 bar N
2 [4]. With the same catalyst, Ru/C, in the presence of THF as the solvent yielded 94.7% DMF at 200 °C under 20 bar H
2 for 2 h
[2].
3. Hydrogenation Reactions with a Transitional Metal Catalyst
To date, most of the catalysts being used in the transformation of HMF into DMF involved precious metals such as Ru, Pt and Pd
[13]. Thus an alternative catalytic system based on non-precious metals (Co, Ni, Cu and Fe) is crucial from the economic point of view since they are cheaper.
Ni and Co are typically active metals for hydrogenation processes since both metals can hydrogenate C=C and C=O bonds
[14][15]. Raney nickel catalyst, for example, has been used in a wide range of hydrogenation reactions, such as in the hydrogenation of nitro compounds, alkenes, carbonyl compounds, nitriles, alkynes and aromatic compounds
[16]. Recently, Iriondo et al. demonstrated that Cu catalyst supported on ZrO
2 has the best selectivity towards DMF among other compared metals such as Pt and Ru
[17]. Ni supported on Co
3O
4 also shows good activity as an additive to Co
3O
4 in converting HMF to DMF, since both of the elements have a good ability to break C–O bonds
[18]. A 76% yield of DMF was achieved under relatively mild reaction conditions (130 °C, 10 bar H
2, 24 h). Kong et al. showed that Ni supported on Al
2O
3 is promising for HMF hydrogenation, with a high yield of DMF, DMTHF and DHMTHF
[14]. The modulation of the surface metal–acid bifunctional site via calcination temperatures and control reaction conditions resulted in a high yield of DMF (91.5%), DMTHF (97.4%) and DHMTHF (96.2%).
Catalysts based on Ni and Co were also used in the chemoselective hydrogenation of furfural. Furfural hydrogenation is quite similar to HMF hydrogenation, apart from having less methyl and a hydroxyl group
[17]. Ni/SiO
2 was reported to have better reactivity than Rh/SiO
2 for the hydrogenation of furfural and selectivity to furfuryl alcohol
[19].
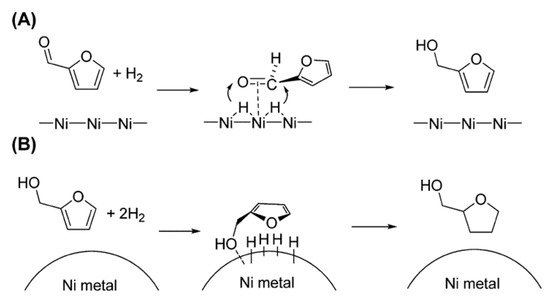
Figure 1 shows the schemes proposed by Nakagawa et al. for the hydrogenation mechanism of furfural and furfural alcohol over Ni/SiO
2 catalyst. Based on the kinetic analysis, they reported that adsorption of furfural on Ni/SiO
2 catalyst is much stronger than that of furfural alcohol (FOL) under hydrogenation conditions, suggesting that furfural is adsorbed at the C=O group
[20].
Figure 1. Proposed mechanisms for the hydrogenation of (
A) furfural and (
B) FOL over Ni/SiO
2 [19].
The use of new and cheap metals for the hydrogenation of HMF to DMF should be explored more since this could be beneficial as an alternative for the precious metal. Apart from the transition metal being used as a monometallic catalyst for hydrogenation, the combination of these metals with other transition metals or noble metals is also getting more attention
[13].
4. Hydrogenation Reactions with Bimetallic Catalysts
Bimetallic catalysts have been receiving a lot of attention in selective oxidation
[21], Fischer–Tropsch
[22] and hydrogenation catalytic
[23] reactions. This is due to positive synergistic effects emerging upon alloying that result in structures and properties that are distinct from those of the pure element. The chemical and physical properties may be tuned by varying the composition and atomic ordering as well as the size of the clusters. In fact, bimetallic metal nanoparticles may display not only magic sizes but also magic compositions at which the alloy presents special stability that may determine the chemical reactivity, especially catalytic activity
[24]. For instance, in benzene hydrogenation, Yoon et al.
[25] synthesised a bimetallic catalyst of Pd-Rh/CNT via a microemulsion method, and it was found that this combination exhibits the highest TOF compared to its monometallic analogues. Another example was shown by Zhu et al., who showed that Ru-Ni/C (0.024 wt% Ru, 1.00 wt% Ni) shows the highest TOF for hydrogenation of benzene compared to monometallic Ni/C and Ru/C
[23]. The authors suggested that this is due to the efficient synergistic effect between Ru, Ni and NiO sites, stemming from the nanostructure of Ru on Ni/NiO nanoparticles. It is also speculated that the additional metals can improve the size and morphology of active particles as well as the catalysts’ selectivity
[26].
In the hydrogenation of glucose to sorbitol, the addition of metalloids (elements that have properties of both metals and non-metals, such as boron, B) as the promoter to Ni demonstrated an improvement in activity. The authors found that the higher activity of this alloy catalyst is due to the combination of the structural features and the surface electronic state
[27]. Extended X-ray absorption fine structure (EXAFS) analyses of the samples revealed that the amorphous catalyst has a lower Ni coordination number and a shorter Ni–Ni bond distance compared to a crystalline catalyst that has been calcined. This was considered to be responsible for the active catalyst, which is beneficial for hydrogenation reactions. Furthermore, the addition of B to Ni makes it electron-rich, and as a consequence, the glucose adsorption through the C=O group is weakened. This means that more H
2 could be adsorbed on the Ni-B catalyst, and hence a higher hydrogenation activity is observed
[27].
Besides improving the reactivity of the reaction, the bimetallic system also can help in reducing the dependence on noble metals by incorporating some non-noble metals, such as Ni and Co. Not only they are cheaper, but in certain molar ratios, they could result in better reactivity than the noble metals themselves
[28].
As for the hydrogenation of bio-derived furan derivatives such as furfural and HMF, many Ni-, Co- or Cu-based bimetallic catalysts have been used
[29]. Recently. Chen et al. demonstrated that carbon-coated Cu-Co bimetallic nanoparticles show excellent performance in selective hydrogenolysis of HMF to DMF, with 99.4% yield of DMF at 180 °C and 50 bar H
2 in 8 h
[30]. X-ray photoelectron spectroscopy (XPS) analysis revealed the coexistence of cobalt oxide species that are responsible for the synergistic effect between cobalt species and copper, while Luo et al. reported that (10 wt%) Pt-Ni alloyed nano-crystals supported on carbon with a ratio of 3:1 exhibit a high yield of DMF compared to other compositions and its monometallic catalysts
[31]. Ni-Fe/CNT (10 wt%) also displayed high selectivity towards DMF and DHMF, depending on the temperature. This was attributed to the formation of Ni-Fe alloys species that is beneficial to the C–O bond cleavage
[32]. The bimetallic non-noble metals were also demonstrated by Giovanni et al. A high yield of the mixture of both DMF and DMTHF was obtained when Cu-Zn nanoalloy was used in HMF hydrogenation
[33]. The authors proposed that the synergistic effect between active CuO sites with Lewis acidic ZnO sites is responsible for the high reactivity based on a previous study
[34].
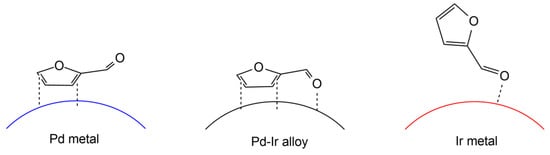
Figure 2 illustrates the adsorption structure of furfural on the catalyst surface, as proposed by Nakagawa et al.
[35]. It was believed that the addition of Ir may promote the adsorption of the C=O site on furfural and weaken the adsorption on the furan ring in the case of Pd-Ir/SiO
2 catalyst. This was demonstrated by comparing the selectivity of furfural and furfuryl alcohol on Pd-Ir alloy to monometallic Pd and Ir. The high reactivity of furan ring hydrogenation was observed on monometallic Pd. In the presence of furfural, the hydrogenation of furfuryl alcohol over Ir was suppressed, suggesting the strong adsorption of C=O on the surface of Ir.
Figure 2. The proposed adsorption structure of furfural on the catalyst surface. Adapted from
[35].
The property of the adsorption of the substrate on the catalyst can be changed by the addition of a secondary metal, particularly an oxophilic metal (electropositive element) such as Sn. Vetere et al.
[19] studied the chemoselective hydrogenation of furaldehyde using monometallic Pt, Ni or Rh and a bimetallic catalyst with Sn supported on SiO
2. They found that the addition of Sn shows significant conversion for Pt and an increase in selectivity towards furfuryl alcohol with Ni.
Figure 3 shows the promotion effect of an oxophilic metal on substrate adsorption at the C=O bond
[36][37][38]. The presence of an oxophilic metal or cation on the noble metal surface induces the hydrogen species formed on the noble metal to be transferred to the C=O bond to achieve selective hydrogenation.
Figure 3. The proposed adsorption structure of furfural on the catalyst surface.
After the recent increased interest in biomass-related transformations, the challenges for the catalysis chemist reside in the complex structure and functionalities of biomass. The challenge in designing catalysts to facilitate the new transformations is to prepare catalysts that can selectively remove functional groups and break specific chemical bonds in the biomass-derived feedstock. Thus, bimetallic catalyst systems seem to be promising due to the synergistic effects between two combination metals.
This synergistic phenomenon can be explained by recent studies by Luo et al.
[31][39] and Chen et al.
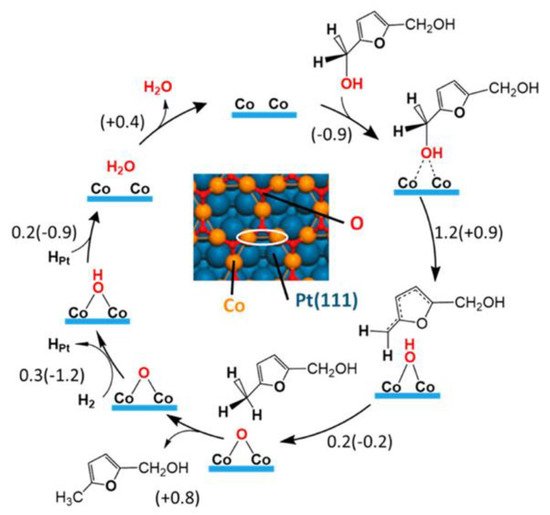
[30]. The monolayer oxide present on the surface interacted weakly with the furan ring to prevent the hydrogenation of the ring and the ring-opening of DMF. At the same time, it acted as an active site for the hydrogenolysis process. The proposed successive hydrogenolysis mechanism of DHMF on Pt-Co alloy is depicted in
Figure 4. DHMF undergoes C–O bond cleavage on a honeycomb edge site consisting of two Co atoms, forming a loosely bound radical and an OH group. Next, an H atom transfers from the OH group to the radical, yielding MFA and a chemisorbed oxygen atom. The second hydroxymethyl group undergoes similar C–O scission, forming DMF as the final product.
Figure 4. Reaction mechanism of DHMF hydrodeoxygenation to MFA on the Co
3O
2/Pt(111) surface based on DFT calculation. Reproduced from
[39] by permission of American Chemical Society.
5. The Role of a Support
Catalyst support also plays an important role in the selectivity of hydrogenation reactions. The supports are usually metal oxides or carbon with the goal of maximising the specific surface area, thus giving better dispersion of the active phase. The most common support includes various types of silica, alumina and carbon. However, the use of microporous support typically involves mass transfer issues due to diffusion limitation. In porous catalyst particles, the reacting molecules diffuse first through the fluid film surrounding the particle surface and then into the pores of the catalyst to the active sites. Similarly, the reaction products diffuse out of the catalyst grains. As an outcome of the pore diffusion in the most common reaction kinetics, the reaction rates inside the pore are lower than with the concentration level of the main bulk.
Carbon materials have been one of the most widely studied supports for catalyst preparation owing to their advantages
[40]. Other than being low in cost, carbon also gives better dispersion due to a high surface area compared to other supports, such as Al
2O
3 and SiO
2. Moreover, the carbon surface is relatively inert, preventing any unwanted reactions catalysed by the support surface or the reaction of the support with the active phase. Most importantly, carbon can minimise poisoning issues due to its hydrophobic nature. For example, it leads to a weaker interaction between the catalyst and the solvent. However, the chemical nature of their surface can be modified chemically to decrease the hydrophobic character by oxidising treatment
[40]. For example, treatment with hydrogen peroxide and nitric acid introduces oxygen surface groups, which are responsible for the improvement of the hydrophilic character of the carbon surface.
Priecel et al. demonstrated that CNTs as support of Ru improve DMF yield and shorten the reaction time from 3 h to 1 h compared to carbon as support. This owing to the superior accessibility of pores in carbon nanotubes, together with an electronic promotional effect in the carbon nanotubes, appears to be responsible for the superior activity of the catalysts supported on carbon nanotubes
[41].
Ricardo et al. reported that Ru supported on materials with a high isoelectric point (basic), such as ceria, magnesia-zirconia and ɤ-alumina, results in a high yield of DHMTHF as compared to a low-isoelectric-point (acidic) support, such as SiO
2, for selective hydrogenation of HMF
[42]. This demonstrates that the basicity of the catalyst support favours the formation of ring hydrogenation of DHMF to DHMTHF. In contrast, the acidic support favours the formation of ring-opening products, such as 1,2,5-hexanetriol.
The effect of different supports on a palladium catalyst for the hydrogenation of HMF was studied by Cai et al.
[43]. Acidic Pd/ɤ-Al
2O
3 and Pd/SiO
2 show a similar activity as that of Pd/C, apart from the selectivity towards DHMTF, which is higher than that of Pd/C, suggesting that the acidity of the support influences the product’s selectivity. When the reaction is prolonged to 3 h, the final product is mainly DHMTHF due to the saturation of the C=C bond in DHMF (
Scheme 2). As for Pd/TiO
2, DHMF is the main product even when the reaction time is prolonged. It is speculated that by dispersing the metal on the reductive support (TiO
2), the metal catalyst demonstrates the potential for selectively hydrogenating the carbonyl group without affecting the C=C bond 89]. In the case of Pd/HT catalyst, the basic HT support is found to restrain the dehydration reaction and inhibits the hydrogenation activity of Pd. It is also reported that HT could induce ring-opening, C–O dissociation and other side reactions
[44]. An extensive review on the effect of the support used and the kinetic modelling of HMF transformation into DMF has been performed by Maki et al.
[45] and Esteves et al.
[46].
6. The Role of Solvents
The medium of reaction also plays an important role in determining the reactivity or the product yield in a chemical reaction, especially the hydrogenation of HMF
[47]. Usually, a solvent like water is preferable since it is a green solvent and no extra precaution is needed to handle it as a waste. However, not all reactions are suitable with water as the solvent. In general, a solvent can be divided into three different categories: polar protic, polar aprotic and non-polar. Polar protic solvents often display hydrogen bonding and have acidic hydrogen. These solvents have a high dielectric constant and polarity for instant water, formic acid, methanol, butanol and propanol. These types of solvents are most likely to participate in the reaction. Polar aprotic solvents lack hydrogen bonds and have moderate-to-high dielectric constants. They do not participate in the reaction, as they are relatively free in solution, making them more reactive. Common examples of such solvents are acetone, acetonitrile, THF and DMSO. Non-polar solvents are solvents such as hexane, benzene and toluene that have low dielectric constants and are not good solvents for a charged species.
A recent study by Chatterjee et al.
[48] showed that a solvent with negative delta (δ) values that is capable of accepting electrons shows higher conversion of HMF. However, as the δ value increased, the conversion of HMF decreased. The authors suggested that solvent adsorption leads to the partial blocking of metal active sites. In addition, a neutral medium was preferred over a basic or an acidic medium. Water was also found to be superior to other organic solvents used for the conversion of HMF and the production of DHMF
[44].
The study of the effect of solution-phase acidity on the selectivity for the hydrogenation of HMF was performed by Alamillo et al.
[42]. Treatment of the HMF feed with resin led to an increase of over 20% in the selectivity of DHMTHF using Ru catalyst. The treatment with resin resulted in an increase in pH, and the increment in pH suggested that the minor impurities of acid mixed with HMF decrease the selectivity to DHMTHF. The influence of the addition of specific types of acid on the hydrogenation of HMF was also studied
[42]. Levulinic acid and H
2SO
4 were added to the reaction mixture, and the addition of levulinic acid led to a decrease in DHMTHF yield, while H
2SO
4 resulted in a significant decline in DHMTHF selectivity from 76% to 9%. It was suggested that HMF and DHMF undergo acid-catalysed degradation, thus leading to low selectivity
[42].
The study of the hydrogenation of HMF using different solvents was also carried out by Alamillo et al. using a water-1-butanol biphasic system, water, a mixture of THF and water and THF-alcohol
[42]. It was found that HMF and DHMF are completely converted in each of the reactions. The selectivity of DHMTHF decreased when pure water was used, indicating the presence of additional degradation pathways in the presence of water. Detailed reviews on the effect of solvents were discussed by Wang et al.
[49].
7. Conclusions
Selective hydrogenation of HMF to DMF has been extensively explored by many researchers, and some of them were able to achieve high conversion as well as a high yield of DMF under certain reaction conditions. However, the main challenge is to reduce the operational cost by using cost-effective catalysts as an alternative to the expensive noble metals. Overall, the approach of using bifunctional catalysts is an important direction for catalysis in general and particularly for reactions that require significantly different types of elementary steps as part of an overall reaction. For this reason, efforts to use rational design of interfaces will become important for supplementing the promising initial work in demonstrating bifunctional catalysis for catalytic transformations of biomass-derived 5-hydroxymethylfurfural to a potential liquid fuel, 2,5-dimethylfuran. Apart from the different types of metal, it was also observed that reaction conditions, namely the type of solvent, H2 donor, reaction temperature, reaction time and H2 pressure, play an important role in influencing the HMF conversion and DMF yield.
Future studies should focus on more variety of inexpensive metal combinations as an alloy and sustainable support without compromising the high yield of DMF. For example, the combination of non-noble metals, such as Ni, Co, Cu and Fe, would be interesting since Ni and Co are capable of converting HMF to DMF, although specific metal ratios and loading are crucial in resulting in a better catalyst. A kinetic study would be helpful to learn the adsorption behaviour of HMF and its intermediates, which would provide some insights into the principles of selectivity control that can guide catalyst selection.