Hypertension is one of the major risk factors for arteriosclerosis. Anti-hypertensive peptides derived from animal proteins, such as milk, eggs and fish, are well studied. Anti-hypertensive peptides were also identified from plant proteins such as soybeans. Rice bran, a byproduct of white rice polishing, is rich in protein and its high protein efficiency ratio is well known. This review discusses the anti-hypertensive peptides identified from rice bran protein and their mechanisms. Also, we described protease-digested rice bran from which functional peptides were not isolated.
- rice bran
- peptide
- anti-hypertension
- ACE inhibitory activity
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
1. Introduction
It is said that Hippocrates stated, “Let food be thy medicine and medicine be thy food.” [1]. Over a thousand years later, in the eighteenth century, Antoine Laurent Lavoisier demonstrated that respiration is a form of slow combustion [2]. Lavoisier thought food was burned to sustain life and that heat was released as a result. However, the components of food, such as carbohydrates, lipids and proteins, were not recognized at that time. These three components were identified in the nineteenth century. Digestion and absorption of these nutrients were also identified in this era [3]. During the late nineteenth to the twentieth century, other nutrients were found, such as vital amino acids and vitamins. In 1882, Kanehiro Takaki reported that a vegetable- and protein-rich diet reduced the risk of beriberi and in 1910, Umetaro Suzuki also reported that oryzanine (vitamin B1) extracted from rice bran cured beriberi [4,5]. Nutritional science in this era revealed the relationship between the nutrient components and life-related diseases, especially deficiency disorder. In the late twentieth century, the most concerning nutrient problem was the shift from starvation to satiation. Growing public awareness of the need to prevent metabolic syndromes, such as diabetes and hypertension, improved the quality of life. Peptides have attracted attention to overcome this problem. Peptides are short chains of amino acids connected by peptide bonds. Endogenous peptides exert unique physiological functions due to differences in amino acid sequences. For example, insulin was isolated by Frederick Banting and Charles Best in 1921, and its amino acid sequence was clarified by Frederick Sanger a few decades later [6,7]. Food-derived bioactive peptides were also isolated, such as β-casomorphin from casein peptone by Brantl et al., in 1979 [8]. Animal proteins, such as milk casein, fish protein and egg protein, were well studied as a source of bioactive peptides [9–11]. Plant proteins were also focused on as an origin of bioactive peptides such as anti-hypertensive peptides [12].
Hypertension is a key risk factor for cardiovascular disease, which affects one billion people worldwide. Control of blood pressure was a major issue until the 1940s. Franklin D. Roosevelt died from a hypertensive cerebral hemorrhage and his blood pressure was reported to be over 300 mmHg [13]. After 1950, anti-hypertensive drugs, such as α blockers, diuretics, calcium channel blockers and angiotensin I-converting enzyme (ACE, EC 3.4.15.1) inhibitors, were discovered [14,15]. ACE inhibitory peptides were first found from snake venom in the early 1970s [16,17]. ACE inhibitors from food proteins were first reported in 1979 by Oshima et al. [18]. Of note, this was the same year that opioid peptides derived from food were reported, as described above. ACE inhibitory peptides derived from food proteins have been used for foods for specific health use (FOSHU). Most of the ACE inhibitory peptides derived from food proteins are cleaved by protease digestion of protein-rich food material.
Rice (Oryza sativa), one of the major grains, serves as the staple food for almost half of the human population, and it is usually consumed in a polished form [19]. Rice bran, a byproduct of white rice processing, is rich in protein [20–22]. Approximately 10–20% of rice bran is protein, whereas endosperm contains only 6–8% protein [22]. The high protein efficiency ratio—defined as the ratio of protein that contributes to body growth [23]—of rice bran is well known [24]. As described above, rice bran protein is well studied but not well utilized. To reduce food waste, expanding the use of rice bran may be useful.
2. Anti-Hypertensive Peptides Isolated from Rice Bran Protein
2.1. Leu-Arg-Ala
In general, protein-rich food materials are digested by proteases or fermented by microorganisms, such as Lactobacillales, to produce anti-hypertensive peptides [25]. Several anti-hypertensive peptides are isolated from protease-digested rice bran.
Shobako identified the novel anti-hypertensive peptide Leu-Arg-Ala (LRA) from thermolysin-digested rice bran [26]. Its strong anti-hypertensive effects and vasodilating activity were previously reported [27]. Orally administered LRA demonstrated anti-hypertensive effects by Spontaneously Hypertensive Rat (SHR) examination and its minimal effective dose was 0.25 mg/kg (Figure 1A), which is the most potent anti-hypertensive peptide derived from rice protein.
LRA exhibited potent vasorelaxing activity in the mesenteric artery isolated from SHRs, its half maximal effective concentration (EC50) value was 0.1 μM (Figure 1B); however, its ACE inhibitory activity was not as high (IC50 = 62 μM). EC50 values of food-derived vasorelaxant peptides, such as Arg-Ile-Tyr (Rapakinin) and Ile-His-Arg-Phe (IHRF), are 5.1 μM and 0.57 μM, respectively [28,29]. The vasodilating activity of LRA is the most potent among vasorelaxant peptides identified from grains to date. The vasorelaxing effects of LRA were inhibited by the nitric oxide synthase (NOS) inhibitor, NG-nitro-L-arginine methyl ester hydrochloride (L-NAME), and NO-sensitive guanylyl cyclase inhibitor, 1H-[1,2,4] oxadiazolo [4,3-a] quinoxaline-1-one (ODQ) (Figure 1C,D). Furthermore, vasodilation by LRA was not observed in the endothelial-removed mesenteric artery (Figure 1E) and its anti-hypertensive effects were inhibited by L-NAME in an in vivo study (Figure 2). These results suggest that an NO-mediated pathway is the main mechanism of the anti-hypertensive activity of LRA.
The NO-mediated vasodilation pathway is well studied and a typical pathway is shown in Figure 3. Endogenous peptide hormones, such as angiotensin (1–7) and bradykinin, induce NO production through the PI3K/Akt/endothelial NOS (eNOS) pathway [30–33]. LRA also promotes eNOS phosphorylation, but LRA did not promote the phosphorylation of Akt in HUVEC cells, an endothelial cell model. The vasodilation activity of LRA was not inhibited by wortmannin (PI3K inhibitor) or HOE140 (BR2 inhibitor) [27]. Thus, factors upstream of NO production may be different from ang (1–7) and bradykinin. Other food-derived vasorelaxing peptides function via cholecystokinin (CCK) or prostaglandin I2 (PGI2) pathways, but LRA-induced vasorelaxation was not inhibited by lorglumide or indomethacin, a CCK antagonist and cyclooxygenase (COX) inhibitor, respectively [28,34]. Thus, LRA may relax the mesenteric artery via a novel pathway coupled to the NO system. This suggests that food-derived exogenous bioactive peptides, including LRA, and endogenous ligands can help reveal novel pathways in the cardiovascular system.
The origin of LRA was identified [26]; it was cleaved from a vicilin-like storage protein belonging to the cupin superfamily protein, one of the major rice bran proteins (Figure 4).
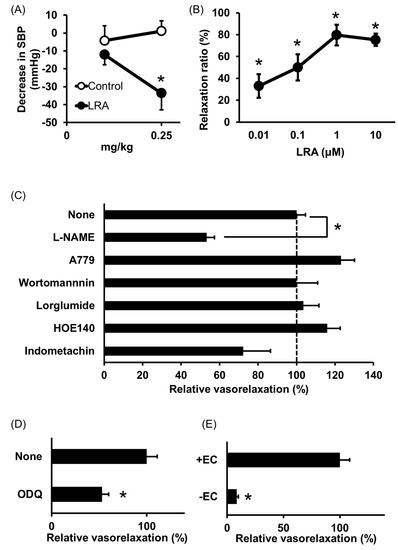
Figure 1. Anti-hypertensive effect of Leu-Arg-Ala (LRA). (A) Minimum effective dose was determined by in vivo study. Peptide samples were administered as a solution in saline. Each point represents the mean reduction in the systolic blood pressure (SBP) of SHRs and the vertical bars indicate the standard errors. * p < 0.05 indicates a significant difference compared with the control group, which was administered the same volume of saline (N = 5–10). These figures were modified and quoted from those previously reported by the author [26]. (B) Dose-dependency of the vasorelaxing activity of LRA. The peptide sample was applied for each concentration alone. The relaxation ratio was calculated using vasorelaxation with 100 μM papaverine as 100%. * p < 0.05, vs. water control group. (C,D) Effects of vasorelaxing pathway blockers that function in the endothelial layer (C) or vascular smooth muscle layer (D) on LRA-induced vasorelaxation. LRA = 10 μM, N = 4–8, * p < 0.05, vs. LRA alone. (E) Endothelial layer-removed samples (EC-) were also assessed. LRA = 10 μM, N = 3–9, * p < 0.05, vs. LRA alone. These figures were modified and quoted from those previously reported by the author [27]. SHR, Spontaneously hypertensive rats; L-NAME, N(G)-nitro-L-arginine methyl ester hydrochloride; ODQ, 1H-[1,2,4] oxadiazolo [4,3-a] quinoxalin-1-on
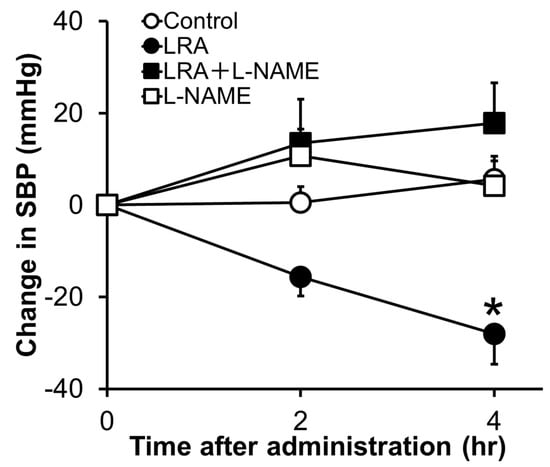
Figure 2. Effects of L-NAME, an NOS inhibitor, on the anti-hypertensive activity of LRA after oral administration in SHRs. L-NAME (20 mg/kg) was administrated just before the oral administration of LRA (1.0 mg/kg) or saline. The Y-axis represents the change in SBP from the beginning of the examination. Values are the mean ± SEM (N= 8–10). * p < 0.05, vs. control group. These figures were modified and quoted from those previously reported by the author [27]. L-NAME, N(G)-nitro-L-arginine methyl ester hydrochloride.
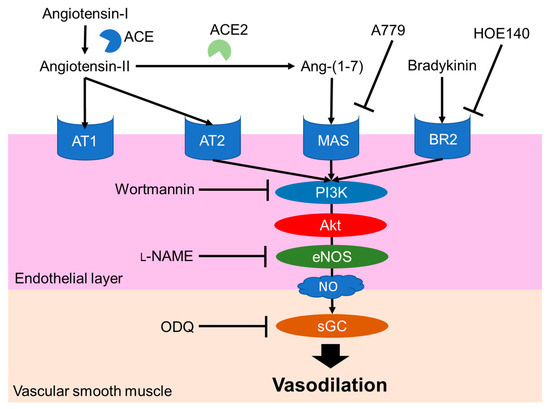
Figure 3. Major NO-mediated vasodilation pathways. Endogenous peptide hormones activate NO production by the phosphorylation of PI3K, Akt and eNOS. sGC, soluble guanylate cyclases; NO, nitric oxide; eNOS, endothelial nitric oxide synthase; Akt, protein kinase b; BR2, Bradykinin receptor B2, L-NAME, N(G)-nitro-L-arginine methyl ester hydrochloride
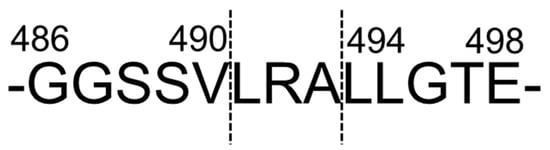
Figure 4. Predicted cleavage site of LRA from vicilin-like storage protein. These figures were modified and quoted from those previously reported by the author [26].
2.2. Tyr-Tyr
Tyr-Tyr (YY) was also identified from thermolysin-digested rice bran as an anti-hypertensive peptide [26]; orally administered YY reduced the blood pressure at 0.5 mg/kg in SHRs (Figure 5A). Its high ACE inhibitory activity was also confirmed by an IC50 = 16 μM.
ACE is in a membrane-bound form in endothelial cells, neuroepithelial cells and the brain [35]. A soluble form was also reported, and is present in blood and different body fluids. ACE is a dipeptidyl carboxypeptidase that catalyzes the conversion of angiotensin I (Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu) to His-Leu and angiotensin II (Asp-Arg-Val-Tyr-Ile-His-Pro-Phe), a vasopressor peptide hormone [36]. ACE inhibitors reduce blood pressure by inhibiting ACE. ACE inhibition is a major mechanism of anti-hypertensive peptides derived from food-derived proteins. YY was also identified from protease-digested royal jelly and its high ACE inhibitory activity was previously reported [37].
In addition, the renin inhibitory activity of YY was previously reported [38]. Renin (EC 3.4.23.15) cleaves angiotensinogen to angiotensin-I and renin inhibitors are also used as anti-hypertensive drugs [39]. Renin inhibitory activity may lead to anti-hypertensive effects.
The origin of YY was previously identified [26]. It was cleaved from vicilin-like storage protein at a different site from LRA (Figure 5B).
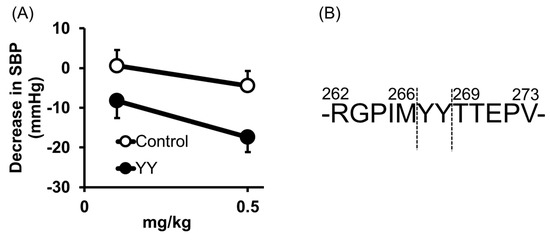
Figure 5. Anti-hypertensive effect of Tyr-Tyr (YY) (A) and predicted cleavage site of YY from vicilin-like storage protein (B). (A) Minimum effective dose was determined by in vivo study. Peptide samples were administered as a solution in saline. Each point represents the mean reduction in SBP of SHRs and vertical bars indicate the standard errors. * p < 0.05 indicates a significant difference compared with the control group, which was administered the same volume of saline (N = 5–10). (B) YY was cleaved from vicilin-like storage protein, at a different site from LRA. These figures were modified and quoted from those previously reported by the author [26]. SBP, systolic blood pressure; SHRs, spontaneously hypertensive rats
2.3. Tyr-Ser-Lys
Tyr-Ser-Lys (YSK) was identified from trypsin-digested rice bran and its ACE inhibitory activity was measured as IC50 = 76 μM [40], being similar to LRA. The molecular docking study revealed that the ACE inhibition of YSK was mainly due to the formation of strong hydrogen bonds with the active pockets of human ACE.
It is well known that peptides exhibiting potent ACE inhibitory activity do not always exert strong anti-hypertensive effects [12]. An in vivo study of this peptide has not been reported and assessment of its anti-hypertensive effects in animal models is expected. However, there are many reports demonstrating that in vitro ACE inhibitory activity and in vivo anti-hypertensive effects are not linked. Further studies are also warranted to identify the cleavage site of this peptide.
References
- Milner, J.A. Functional foods and health promotion. Nutr. 1999, 129, 1395S–1397S, doi:10.1093/jn/129.7.1395S.
- Tan, S.Y.; Hu, M. Antoine-Laurent Lavoisier (1743–1794): Founder of modern chemistry. Singapore Med. J. 2004, 45, 303–304.
- Ryokuerou, S. The backward and future of nutiriology. J. Nutr. Diet. 1973, 31, 219–220, doi:10.5264/eiyogakuzashi.31.219.
- Yoshida, A. The origin of food nutriology. Kagaku To Seibutsu 1984, 22, 583–590, doi:10.1271/kagakutoseibutsu1962.22.583.
- Sugiyama, Y.; Seita, A. Kanehiro Takaki and the control of beriberi in the Japanese Navy. R. Soc. Med. 2013, 106, 332–334, doi:10.1177/0141076813497889.
- Stretton, A.O.W. The first sequence: Fred Sanger and insulin. Genetics 2002, 162, 527–532.
- Karamanou, M. Milestones in the history of diabetes mellitus: The main contributors. World J. Diabetes 2016, 7, 1, doi:10.4239/wjd.v7.i1.1.
- Brantl, V.; Teschemacher, H.; Henschen, A.; Lottspeich, F. Novel opioid peptides derived from casein (beta-casomorphins). I. Isolation from bovine casein peptone. Hoppe Seylers. Z. Physiol. Chem. 1979, 360, 1211–1216, doi:10.1515/bchm2.1979.360.2.1211.
- Hartmann, R.; Meisel, H. Food-derived peptides with biological activity: From research to food applications. Opin. Biotechnol. 2007, 18, 163–169, doi:10.1016/j.copbio.2007.01.013.
- Bhat, Z.F.; Kumar, S.; Bhat, H.F. Bioactive peptides of animal origin: A review. Food Sci. Technol. 2015, 52, 5377–5392, doi:10.1007/s13197-015-1731-5.
- Lee, S.Y.; Hur, S.J. Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem. 2017, 228, 506–517, doi:10.1016/j.foodchem.2017.02.039.
- Guang, C.; Phillips, R.D. Plant food-derived angiotensin i converting enzyme inhibitory peptides. Agric. Food Chem. 2009, 57, 5113–5120, doi:10.1021/jf900494d.
- Moser M. Historical perspectives on the management of hypertension. J Clin Hypertens (Greenwich). 2006 Aug;8(8 Suppl 2):15–20; quiz 39. doi: 10.1111/j.1524-6175.2006.05836.x.
- Laragh, J.H.; Brenner, B.M.; Freis, E.D. Hypertension: Pathophysiology, Diagnosis, and Management, 2nd ed.; Raven Press: New York, NY, USA, 1995.
- Moser, M. Evolution of the treatment of hypertension from the 1940s to JNC V. J. Hypertens. 1997, 10, 2S–8S.
- Ferreira, S.H.; Bartelt, D.C.; Greene, L.J. Isolation of bradykinin-potentiating peptides from Bothrops jararaca venom. Biochemistry 1970, 9, 2583–2593, doi:10.1021/bi00815a005.
- Ondetti, M.A.; Williams, N.J.; Sabo, E.; Pluscec, J.; Weaver, E.R.; Kocy, O. Angiotensin-converting enzyme inhibitors from the venom of Bothrops jararaca. Isolation, elucidation of structure, and synthesis. Biochemistry 1971, 10, 4033–4039, doi:10.1021/bi00798a004.
- Ariyoshi, Y. Angiotensin-converting enzyme inhibitors derived from food proteins. Trends Food Sci. Technol. 1993, 4, 139–144, doi:10.1016/0924-2244(93)90033-7.
- Muthayya, S.; Sugimoto, J.D.; Montgomery, S.; Maberly, G.F. An overview of global rice production, supply, trade, and consumption. NY Acad. Sci. 2014, 1324, 7–14, doi:10.1111/nyas.12540.
- Wattanasiritham, L.; Theerakulkait, C.; Wickramasekara, S.; Maier, C.S.; Stevens, J.F. Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein. Food Chem. 2016, 192, 156–162, doi:10.1016/j.foodchem.2015.06.057.
- Shih, F.F. An update on the use of co-products from the milling of rice in value-added food products. Am. Oil Chem. Soc. 2012, 89, 1–8, doi:10.1007/s11746-011-1941-6.
- Kadowaki, M.; Kubota, M.; Watanabe, R. Physiological multifunctions of rice proteins of endosperm and bran. Nutr. Sci. Vitaminol. (Tokyo) 2019, 65, S42–S47, doi:10.3177/jnsv.65.S42.
- Mansilla, W.D.; Marinangeli, C.P.F.; Cargo-Froom, C.; Franczyk, A.; House, J.D.; Elango, R.; Columbus, D.A.; Kiarie, E.; Rogers, M.; Shoveller, A.K. Comparison of methodologies used to define the protein quality of human foods and support regulatory claims. Physiol. Nutr. Metab. 2020, 45, 917–926, doi:10.1139/apnm-2019-0757.
- Han, S.W.; Chee, K.M.; Cho, S.J. Nutritional quality of rice bran protein in comparison to animal and vegetable protein. Food Chem. 2015, 172, 766–769, doi:10.1016/j.foodchem.2014.09.127.
- Yasunori, N.; Osamu, M.; Toshiaki, T. Decrease of tissue angiotensin I-converting enzyme activity upon feeding sour milk in spontaneously hypertensive rats. Biotechnol. Biochem. 1996, 60, 488–489, doi:10.1080/00021369.1989.10869385.
- Shobako, N.; Ogawa, Y.; Ishikado, A.; Harada, K.; Kobayashi, E.; Suido, H.; Kusakari, T.; Maeda, M.; Suwa, M.; Matsumoto, M.; et al. A novel antihypertensive peptide identified in thermolysin-digested rice bran. Nutr. Food Res. 2018, 62, 1–7, doi:10.1002/mnfr.201700732.
- Shobako, N.; Ishikado, A.; Ogawa, Y.; Sono, Y.; Kusakari, T.; Suwa, M.; Matsumoto, M.; Ohinata, K. Vasorelaxant and antihypertensive effects that are dependent on the endothelial NO system exhibited by rice bran-derived tripeptide. Agric. Food Chem. 2019, 67, 1437–1442, doi:10.1021/acs.jafc.8b06341.
- Yamada, Y.; Iwasaki, M.; Usui, H.; Ohinata, K.; Marczak, E.D.; Lipkowski, A.W.; Yoshikawa, M. Rapakinin, an anti-hypertensive peptide derived from rapeseed protein, dilates mesenteric artery of spontaneously hypertensive rats via the prostaglandin IP receptor followed by CCK1receptor. Peptides 2010, 31, 909–914, doi:10.1016/j.peptides.2010.02.013.
- Kontani, N.; Omae, R.; Kagebayashi, T.; Kaneko, K.; Yamada, Y.; Mizushige, T.; Kanamoto, R.; Ohinata, K. Characterization of Ile-His-Arg-Phe, a novel rice-derived vasorelaxing peptide with hypotensive and anorexigenic activities. Nutr. Food Res. 2014, 58, 359–364, doi:10.1002/mnfr.201300334.
- Santos, R.A.S.; Ferreira, A.J.; Verano-Braga, T.; Bader, M. Angiotensin-converting enzyme 2, angiotensin-(1-7) and Mas: New players of the renin-angiotensin system. Endocrinol. 2013, 216, doi:10.1530/JOE-12-0341.
- Xia, N.; Förstermann, U.; Li, H. Effects of resveratrol on eNOS in the endothelium and the perivascular adipose tissue. NY Acad. Sci. 2017, 1403, 132–141, doi:10.1111/nyas.13397.
- Sharma, R.; Randhawa, P.K.; Singh, N.; Jaggi, A.S. Bradykinin in ischemic conditioning-induced tissue protection: Evidences and possible mechanisms. J. Pharmacol. 2015, 768, 58–70, doi:10.1016/j.ejphar.2015.10.029.
- Peiró, C.; Vallejo, S.; Gembardt, F.; Palacios, E.; Novella, S.; Azcutia, V.; Rodríguez-Mañas, L.; Hermenegildo, C.; Sánchez-Ferrer, C.F.; Walther, T. Complete blockade of the vasorelaxant effects of angiotensin-(1-7) and bradykinin in murine microvessels by antagonists of the receptor Mas. Physiol. 2013, 591, 2275–2285, doi:10.1113/jphysiol.2013.251413.
- Hirota, T.; Nonaka, A.; Matsushita, A.; Uchida, N.; Ohki, K.; Asakura, M.; Kitakaze, M. Milk casein-derived tripeptides, VPP and IPP induced NO production in cultured endothelial cells and endothelium-dependent relaxation of isolated aortic rings. Heart Vessels 2011, 26, 549–556, doi:10.1007/s00380-010-0096-y.
- Brown, N.J.; Vaughan, D.E. Angiotensin-converting enzyme inhibitors. Circulation 1998, 97, doi:10.1016/B978-1-4377-2766-1.00020-X.
- He, H.L.; Liu, D.; Ma, C.B. Review on the Angiotensin-I-Converting Enzyme (ACE) inhibitor peptides from marine proteins. Biochem. Biotechnol. 2013, 169, 738–749, doi:10.1007/s12010-012-0024-y.
- Maruyama, H.; Tokunaga, K.; Suzuki, K.M.; Yoshida, C.; Futamura, Y.; Araki, Y.; Mishima, S. Purification and identification of angiotensin I-converting enzyme inhibitory peptides from royal jelly treated with protease. Nippon Shokuhin Kagaku Kogaku Kaishi 2003, 50, 310–315.
- Sultana, A.; Nabi, A.H.M.N.; Nasir, U.M.; Maruyama, H.; Suzuki, K.M.; Mishima, S.; Suzuki, F. A dipeptide YY derived from royal jelly proteins inhibits renin activity. J. Mol. Med. 2008, 21, 677–681, doi:10.3892/ijmm.21.6.677.
- Pantzaris, N.-D.; Karanikolas, E.; Tsiotsios, K.; Velissaris, D. Renin inhibition with aliskiren: A decade of clinical experience. Clin. Med. 2017, 6, 61, doi:10.3390/jcm6060061.
- Wang, X.; Chen, H.; Fu, X.; Li, S.; Wei, J. A novel antioxidant and ACE inhibitory peptide from rice bran protein: Biochemical characterization and molecular docking study. LWT Food Sci. Technol. 2017, 75, 93–99, doi:10.1016/j.lwt.2016.08.047.
This entry is adapted from the peer-reviewed paper 10.3390/nu12103060
