Salicylic acid (SA) is a plant hormone that has been implicated in processes not limited to plant growth, development, and responses to environmental stress. This review summarize the various roles and functions of SA in mitigating abiotic stresses to plants, including heating, chilling, salinity, metal toxicity, drought, ultraviolet radiation, etc. Consistent with its critical roles in plant abiotic tolerance, the gaps in the literature with regard to the complex signalling network between SA and reactive oxygen species, ABA, Ca2+, and nitric oxide were identified. Furthermore, the molecular mechanisms underlying signalling networks that control development and stress responses in plants, and underscore prospects for future research on SA concerning abiotic-stressed plants were also discussed.
- abiotic stress
- reactive oxygen species
- salicylic acid
1. Functions of SA in Mitigating Abiotic Stresses
1.1. Heat
1.2. Chilling
1.3. Salinity
1.4. Metal Toxicity
1.5. Other Stresses
1.5.1. Drought
1.5.2. Ozone
1.5.3. Pesticide
1.5.4. Ultraviolet Radiation
2. Possible Mechanisms of SA in Mitigating Abiotic Stresses
2.1. Redox Signalling
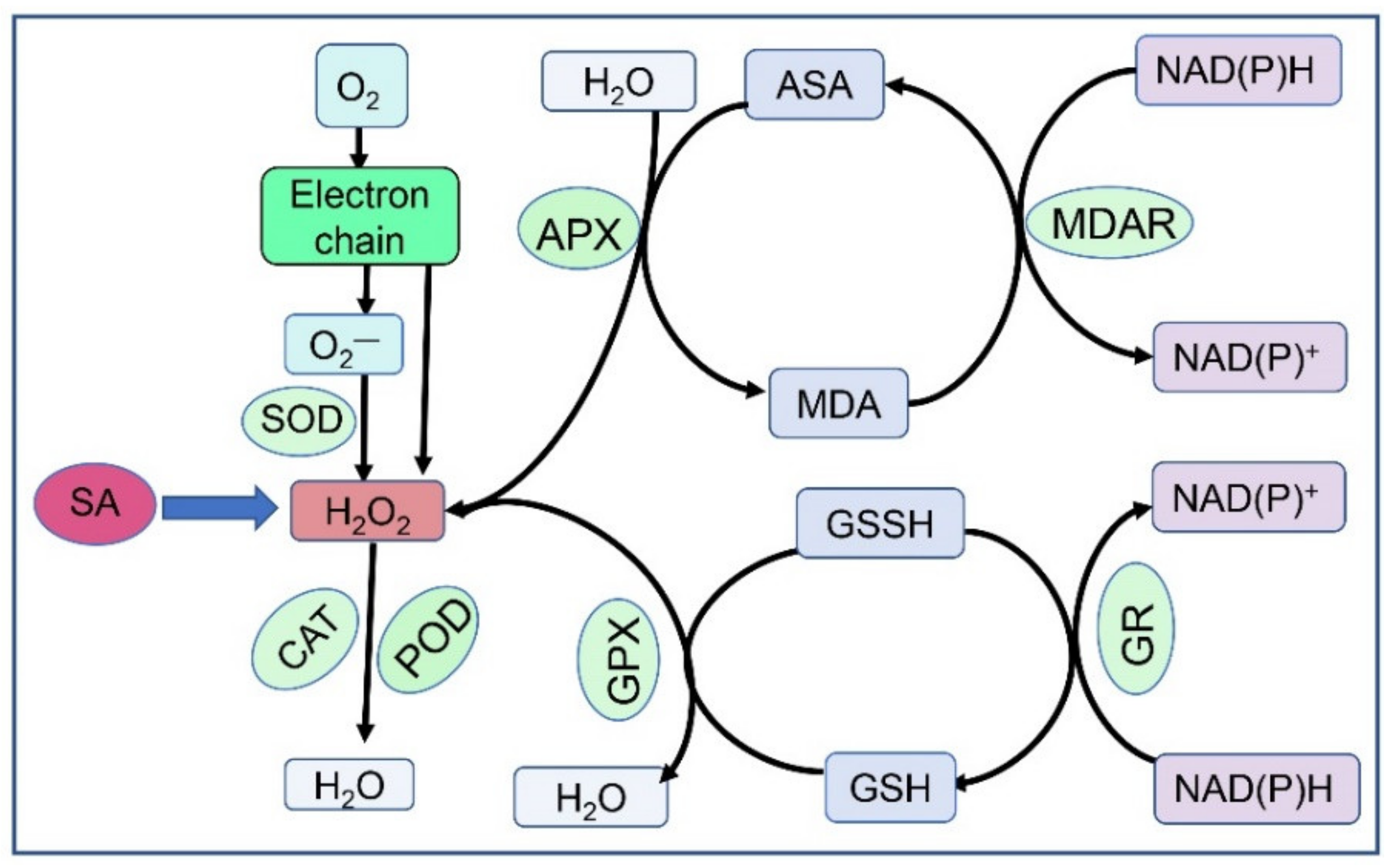
2.2. Cross-Talk with Other Plant Hormones
2.3. Mitogen-Activated Protein Kinase
This entry is adapted from the peer-reviewed paper 10.3390/life12060886
References
- Bita, C.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273.
- Yang, J.; Duan, L.; He, H.; Li, Y.; Li, X.; Liu, D.; Wang, J.; Jin, G.; Huang, S. Application of Exogenous KH2PO4 and Salicylic Acid and Optimization of the Sowing Date Enhance Rice Yield Under High-Temperature Conditions. J. Plant Growth Regul. 2022, 41, 1–15.
- Pan, Q.; Zhan, J.; Liu, H.; Zhang, J.; Chen, J.; Wen, P.; Huang, W. Salicylic acid synthesized by benzoic acid 2-hydroxylase participates in the development of thermotolerance in pea plants. Plant Sci. 2006, 171, 226–233.
- Dat, J.F.; Foyer, C.H.; Scott, I.M. Changes in salicylic acid and antioxidants during induced thermotolerance in mustard seedlings. Plant Physiol. 1998, 118, 1455–1461.
- Larkindale, J.; Hall, J.D.; Knight, M.R.; Vierling, E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 2005, 138, 882–897.
- Wang, L.-J.; Li, S.-H. Thermotolerance and related antioxidant enzyme activities induced by heat acclimation and salicylic acid in grape (Vitis vinifera L.) leaves. Plant Growth Regul. 2006, 48, 137–144.
- Widiastuti, A.; Yoshino, M.; Hasegawa, M.; Nitta, Y.; Sato, T. Heat shock-induced resistance increases chitinase-1 gene expression and stimulates salicylic acid production in melon (Cucumis melo L.). Physiol. Mol. Plant Pathol. 2013, 82, 51–55.
- Čajánek, M.; Štroch, M.; Lachetova, I.; Kalina, J.; Spunda, V. Characterization of the photosystem II inactivation of heat-stressed barley leaves as monitored by the various parameters of chlorophyll a fluorescence and delayed fluorescence. J. Photochem. Photobiol. B Biol. 1998, 47, 39–45.
- Wassie, M.; Zhang, W.; Zhang, Q.; Ji, K.; Cao, L.; Chen, L. Exogenous salicylic acid ameliorates heat stress-induced damages and improves growth and photosynthetic efficiency in alfalfa (Medicago sativa L.). Ecotoxicol. Environ. Saf. 2020, 191, 110206.
- Shi, Q.; Bao, Z.; Zhu, Z.; Ying, Q.; Qian, Q. Effects of different treatments of salicylic acid on heat tolerance, chlorophyll fluorescence, and antioxidant enzyme activity in seedlings of Cucumis sativa L. Plant Growth Regul. 2006, 48, 127–135.
- Wang, L.-J.; Fan, L.; Loescher, W.; Duan, W.; Liu, G.-J.; Cheng, J.-S.; Luo, H.-B.; Li, S.-H. Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol. 2010, 10, 34.
- Afzal, I.; Akram, M.; Rehman, H.; Rashid, S.; Basra, S. Moringa leaf and sorghum water extracts and salicylic acid to alleviate impacts of heat stress in wheat. S. Afr. J. Bot. 2020, 129, 169–174.
- Jahan, M.S.; Wang, Y.; Shu, S.; Zhong, M.; Chen, Z.; Wu, J.; Sun, J.; Guo, S. Exogenous salicylic acid increases the heat tolerance in Tomato (Solanum lycopersicum L.) by enhancing photosynthesis efficiency and improving antioxidant defense system through scavenging of reactive oxygen species. Sci. Hortic. 2019, 247, 421–429.
- Khan, M.I.R.; Iqbal, N.; Masood, A.; Per, T.S.; Khan, N.A. Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal. Behav. 2013, 8, e26374.
- Liu, Y.; Zhang, J.; Liu, H.; Huang, W. Salicylic acid or heat acclimation pre-treatment enhances the plasma membrane-associated ATPase activities in young grape plants under heat shock. Sci. Hortic. 2008, 119, 21–27.
- Larkindale, J.; Knight, M.R. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002, 128, 682–695.
- Clarke, S.M.; Mur, L.A.; Wood, J.E.; Scott, I.M. Salicylic acid dependent signaling promotes basal thermotolerance but is not essential for acquired thermotolerance in Arabidopsis thaliana. Plant J. 2004, 38, 432–447.
- Cronjé, M.J.; Bornman, L. Salicylic acid influences Hsp70/Hsc70 expression in Lycopersicon esculentum: Dose-and time-dependent induction or potentiation. Biochem. Biophys. Res. Commun. 1999, 265, 422–427.
- Snyman, M.; Cronjé, M. Modulation of heat shock factors accompanies salicylic acid-mediated potentiation of Hsp70 in tomato seedlings. J. Exp. Bot. 2008, 59, 2125–2132.
- Chang, P.-F.L.; Jinn, T.-L.; Huang, W.-K.; Chen, Y.; Chang, H.-M.; Wang, C.-W. Induction of a cDNA clone from rice encoding a class II small heat shock protein by heat stress, mechanical injury, and salicylic acid. Plant Sci. 2007, 172, 64–75.
- Liu, H.-T.; Huang, W.-D.; Pan, Q.-H.; Weng, F.-H.; Zhan, J.-C.; Liu, Y.; Wan, S.-B.; Liu, Y.-Y. Contributions of PIP2-specific-phospholipase C and free salicylic acid to heat acclimation-induced thermotolerance in pea leaves. J. Plant Physiol. 2006, 163, 405–416.
- Li, Z.-G.; Xie, L.-R.; Li, X.-J. Hydrogen sulfide acts as a downstream signal molecule in salicylic acid-induced heat tolerance in maize (Zea mays L.) seedlings. J. Plant Physiol. 2015, 177, 121–127.
- Dinler, B.; Demir, E.; Kompe, Y. Regulation of auxin, abscisic acid and salicylic acid levels by ascorbate application under heat stress in sensitive and tolerant maize leaves. Acta Biol. Hung. 2014, 65, 469–480.
- Wang, L.-J.; Li, S.-H. Salicylic acid-induced heat or cold tolerance in relation to Ca2+ homeostasis and antioxidant systems in young grape plants. Plant Sci. 2006, 170, 685–694.
- Janda, T.; Szalai, G.; Tari, I.; Paldi, E. Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta 1999, 208, 175–180.
- Ansari, O.; Sharif-Zadeh, F. Does Gibberelic acid (GA), Salicylic acid (SA) and Ascorbic acid (ASc) improve Mountain Rye (Secale montanum) seeds germination and seedlings growth under cold stress. Int. Res. J. Appl. Basic Sci. 2012, 3, 1651–1657.
- Cheng, F.; Lu, J.; Gao, M.; Shi, K.; Kong, Q.; Huang, Y.; Bie, Z. Redox signaling and CBF-responsive pathway are involved in salicylic acid-improved photosynthesis and growth under chilling stress in watermelon. Front. Plant Sci. 2016, 7, 1519.
- Gharib, F.; Hegazi, A. Salicylic acid ameliorates germination, seedling growth, phytohormone and enzymes activity in bean (Phaseolus vulgaris L.) under cold stress. J. Am. Sci. 2010, 6, 675–683.
- Ignatenko, A.; Talanova, V.; Repkina, N.; Titov, A. Exogenous salicylic acid treatment induces cold tolerance in wheat through promotion of antioxidant enzyme activity and proline accumulation. Acta Physiol. Plant. 2019, 41, 1–10.
- Kosová, K.; Prášil, I.T.; Vítámvás, P.; Dobrev, P.; Motyka, V.; Floková, K.; Novák, O.; Turečková, V.; Rolčik, J.; Pešek, B. Complex phytohormone responses during the cold acclimation of two wheat cultivars differing in cold tolerance, winter Samanta and spring Sandra. J. Plant Physiol. 2012, 169, 567–576.
- Mutlu, S.; Karadağoğlu, Ö.; Atici, Ö.; Nalbantoğlu, B. Protective role of salicylic acid applied before cold stress on antioxidative system and protein patterns in barley apoplast. Biol. Plant. 2013, 57, 507–513.
- Scott, I.M.; Clarke, S.M.; Wood, J.E.; Mur, L.A. Salicylate accumulation inhibits growth at chilling temperature in Arabidopsis. Plant Physiol. 2004, 135, 1040–1049.
- Kang, G.; Wang, C.; Sun, G.; Wang, Z. Salicylic acid changes activities of H2O2-metabolizing enzymes and increases the chilling tolerance of banana seedlings. Environ. Exp. Bot. 2003, 50, 9–15.
- Khademi, O.; Ashtari, M.; Razavi, F. Effects of salicylic acid and ultrasound treatments on chilling injury control and quality preservation in banana fruit during cold storage. Sci. Hortic. 2019, 249, 334–339.
- Aghdam, M.S.; Jannatizadeh, A.; Sheikh-Assadi, M.; Malekzadeh, P. Alleviation of postharvest chilling injury in anthurium cut flowers by salicylic acid treatment. Sci. Hortic. 2016, 202, 70–76.
- Luo, Z.; Wu, X.; Xie, Y.; Chen, C. Alleviation of chilling injury and browning of postharvest bamboo shoot by salicylic acid treatment. Food Chem. 2012, 131, 456–461.
- Siboza, X.I.; Bertling, I.; Odindo, A.O. Salicylic acid and methyl jasmonate improve chilling tolerance in cold-stored lemon fruit (Citrus limon). J. Plant Physiol. 2014, 171, 1722–1731.
- Siboza, X.I.; Bertling, I.; Odindo, A.O. Enzymatic antioxidants in response to methyl jasmonate and salicylic acid and their effect on chilling tolerance in lemon fruit . Sci. Hortic. 2017, 225, 659–667.
- Zhang, Y.; Zhang, M.; Yang, H. Postharvest chitosan-g-salicylic acid application alleviates chilling injury and preserves cucumber fruit quality during cold storage. Food Chem. 2015, 174, 558–563.
- Ge, W.; Zhao, Y.; Kong, X.; Sun, H.; Luo, M.; Yao, M.; Wei, B.; Ji, S. Combining salicylic acid and trisodium phosphate alleviates chilling injury in bell pepper (Capsicum annuum L.) through enhancing fatty-acid desaturation efficiency and water retention. Food Chem 2020, 327, 127057.
- Wang, L.; Chen, S.; Kong, W.; Li, S.; Archbold, D.D. Salicylic acid pretreatment alleviates chilling injury and affects the antioxidant system and heat shock proteins of peaches during cold storage. Postharvest Biol. Technol. 2006, 41, 244–251.
- Cao, S.; Hu, Z.; Zheng, Y.; Lu, B. Synergistic effect of heat treatment and salicylic acid on alleviating internal browning in cold-stored peach fruit. Postharvest Biol. Technol. 2010, 58, 93–97.
- Sayyari, M.; Castillo, S.; Valero, D.; Díaz-Mula, H.M.; Serrano, M. Acetyl salicylic acid alleviates chilling injury and maintains nutritive and bioactive compounds and antioxidant activity during postharvest storage of pomegranates. Postharvest Biol. Technol. 2011, 60, 136–142.
- Sayyari, M.; Babalar, M.; Kalantari, S.; Serrano, M.; Valero, D. Effect of salicylic acid treatment on reducing chilling injury in stored pomegranates. Postharvest Biol. Technol. 2009, 53, 152–154.
- Luo, Z.; Chen, C.; Xie, J. Effect of salicylic acid treatment on alleviating postharvest chilling injury of ‘Qingnai’plum fruit. Postharvest Biol. Technol. 2011, 62, 115–120.
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of plant responses and adaptation to soil salinity. Innovation 2020, 1, 100017.
- Wang, Y.; Mopper, S.; Hasenstein, K.H. Effects of salinity on endogenous ABA, IAA, JA, and SA in Iris hexagona. J. Chem. Ecol. 2001, 27, 327–342.
- Molina, A.; Bueno, P.; Marín, M.C.; Rodríguez-Rosales, M.P.; Belver, A.; Venema, K.; Donaire, J.P. Involvement of endogenous salicylic acid content, lipoxygenase and antioxidant enzyme activities in the response of tomato cell suspension cultures to NaCl. N. Phytol. 2002, 156, 409–415.
- Hamayun, M.; Khan, S.A.; Khan, A.L.; Shinwari, Z.K.; Hussain, J.; Sohn, E.-Y.; Kang, S.-M.; Kim, Y.-H.; Khan, M.A.; Lee, I.-J. Effect of salt stress on growth attributes and endogenous growth hormones of soybean cultivar Hwangkeumkong. Pak. J. Bot. 2010, 42, 3103–3112. Available online: https://www.pakbs.org/pjbot/PDFs/42(5)/PJB42(5)3103.pdf (accessed on 10 June 2022).
- Kumar, S.; Abass Ahanger, M.; Alshaya, H.; Latief Jan, B.; Yerramilli, V. Salicylic acid mitigates salt induced toxicity through the modifications of biochemical attributes and some key antioxidants in capsicum annuum. Saudi J. Biol. Sci. 2022, 29, 1337–1347.
- Chojak-Koźniewska, J.; Linkiewicz, A.; Sowa, S.; Radzioch, M.; Kuźniak, E. Interactive effects of salt stress and Pseudomonas syringae pv. lachrymans infection in cucumber: Involvement of antioxidant enzymes, abscisic acid and salicylic acid. Environ. Exp. Bot. 2017, 136, 9–20.
- Farhangi-Abriz, S.; Ghassemi-Golezani, K. How can salicylic acid and jasmonic acid mitigate salt toxicity in soybean plants? Ecotoxicol. Environ. Saf. 2018, 147, 1010–1016.
- Gunes, A.; Inal, A.; Alpaslan, M.; Eraslan, F.; Bagci, E.G.; Cicek, N. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J. Plant Physiol. 2007, 164, 728–736.
- Feizi, H.; Moradi, R.; Pourghasemian, N.; Sahabi, H. Assessing saffron response to salinity stress and alleviating potential of gamma amino butyric acid, salicylic acid and vermicompost extract on salt damage. S. Afr. J. Bot. 2021, 141, 330–343.
- Hongna, C.; Leyuan, T.; Junmei, S.; Xiaori, H.; Xianguo, C. Exogenous salicylic acid signal reveals an osmotic regulatory role in priming the seed germination of leymus chinensis under salt-alkali stress. Environ. Exp. Bot. 2021, 188, 104498.
- Ghassemi-Golezani, K.; Farhangi-Abriz, S. Foliar sprays of salicylic acid and jasmonic acid stimulate H+-ATPase activity of tonoplast, nutrient uptake and salt tolerance of soybean. Ecotoxicol. Environ. Saf. 2018, 166, 18–25.
- Jayakannan, M.; Bose, J.; Babourina, O.; Rengel, Z.; Shabala, S. Salicylic acid improves salinity tolerance in Arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. J. Exp. Bot. 2013, 64, 2255–2268.
- Sun, J.; Wang, M.J.; Ding, M.Q.; Deng, S.R.; Liu, M.Q.; Lu, C.F.; Zhou, X.Y.; Shen, X.; Zheng, X.J.; Zhang, Z.K. H2O2 and cytosolic Ca2+ signals triggered by the PM H+-coupled transport system mediate K+/Na+ homeostasis in NaCl-stressed Populus euphratica cells. Plant Cell Environ. 2010, 33, 943–958.
- Liu, T.; Li, T.; Zhang, L.; Li, H.; Liu, S.; Yang, S.; An, Q.; Pan, C.; Zou, N. Exogenous salicylic acid alleviates the accumulation of pesticides and mitigates pesticide-induced oxidative stress in cucumber plants (Cucumis sativus L.). Ecotoxicol. Environ. Saf. 2021, 208, 111654.
- Khan, M.I.R.; Asgher, M.; Khan, N.A. Alleviation of salt-induced photosynthesis and growth inhibition by salicylic acid involves glycinebetaine and ethylene in mungbean (Vigna radiata L.). Plant Physiol. Biochem. 2014, 80, 67–74.
- Shakirova, F.M.; Sakhabutdinova, A.R.; Bezrukova, M.V.; Fatkhutdinova, R.A.; Fatkhutdinova, D.R. Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 2003, 164, 317–322.
- Yadav, V.; Arif, N.; Kováč, J.; Singh, V.P.; Tripathi, D.K.; Chauhan, D.K.; Vaculík, M. Structural modifications of plant organs and tissues by metals and metalloids in the environment: A review. Plant Physiol. Biochem. 2021, 159, 100–112.
- Huihui, Z.; Xin, L.; Zisong, X.; Yue, W.; Zhiyuan, T.; Meijun, A.; Yuehui, Z.; Wenxu, Z.; Nan, X.; Guangyu, S. Toxic effects of heavy metals Pb and Cd on mulberry (Morus alba L.) seedling leaves: Photosynthetic function and reactive oxygen species (ROS) metabolism responses. Ecotoxicol. Environ. Saf. 2020, 195, 110469.
- Jing, C.; Cheng, Z.; Li, L.-P.; Sun, Z.-Y.; Pan, X.-B. Effects of exogenous salicylic acid on growth and H2O2-metabolizing enzymes in rice seedlings under lead stress. J. Environ. Sci. 2007, 19, 44–49.
- Zengin, F. Exogenous treatment with salicylic acid alleviating copper toxicity in bean seedlings. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014, 84, 749–755.
- Zaid, A.; Mohammad, F.; Wani, S.H.; Siddique, K.M. Salicylic acid enhances nickel stress tolerance by up-regulating antioxidant defense and glyoxalase systems in mustard plants. Ecotoxicol. Environ. Saf. 2019, 180, 575–587.
- Islam, F.; Yasmeen, T.; Arif, M.S.; Riaz, M.; Shahzad, S.M.; Imran, Q.; Ali, I. Combined ability of chromium (Cr) tolerant plant growth promoting bacteria (PGPB) and salicylic acid (SA) in attenuation of chromium stress in maize plants. Plant Physiol. Biochem. 2016, 108, 456–467.
- Khalil, R.; Haroun, S.; Bassyoini, F.; Nagah, A.; Yusuf, M. Salicylic acid in combination with kinetin or calcium ameliorates heavy metal stress in Phaseolus vulgaris plant. J. Agric. Food Res. 2021, 5, 100182.
- Kaya, C.; Sarıoglu, A.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. The combined supplementation of melatonin and salicylic acid effectively detoxifies arsenic toxicity by modulating phytochelatins and nitrogen metabolism in pepper plants. Environ. Pollut. 2022, 297, 118727.
- Huybrechts, M.; Cuypers, A.; Deckers, J.; Iven, V.; Vandionant, S.; Jozefczak, M.; Hendrix, S. Cadmium and plant development: An agony from seed to seed. Int. J. Mol. Sci. 2019, 20, 3971.
- Guo, B.; Liu, C.; Liang, Y.; Li, N.; Fu, Q. Salicylic acid signals plant defence against cadmium toxicity. Int. J. Mol. Sci. 2019, 20, 2960.
- Krantev, A.; Yordanova, R.; Janda, T.; Szalai, G.; Popova, L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J. Plant Physiol. 2008, 165, 920–931.
- Metwally, A.; Finkemeier, I.; Georgi, M.; Dietz, K.-J. Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol. 2003, 132, 272–281.
- Metwally, A.; Safronova, V.I.; Belimov, A.A.; Dietz, K.-J. Genotypic variation of the response to cadmium toxicity in Pisum sativum L. J. Exp. Bot. 2005, 56, 167–178.
- Bandurska, H. The effect of salicylic acid on barley response to water deficit. Acta Physiol. Plant. 2005, 27, 379–386.
- Munne-Bosch, S.; Penuelas, J. Photo-and antioxidative protection, and a role for salicylic acid during drought and recovery in field-grown Phillyrea angustifolia plants. Planta 2003, 217, 758–766.
- Abreu, M.E.; Munné-Bosch, S. Salicylic acid may be involved in the regulation of drought-induced leaf senescence in perennials: A case study in field-grown Salvia officinalis L. plants. Environ. Exp. Bot. 2008, 64, 105–112.
- Arif, Y.; Sami, F.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salicylic acid in relation to other phytohormones in plant: A study towards physiology and signal transduction under challenging environment. Environ. Exp. Bot. 2020, 175, 104040.
- Safari, M.; Mousavi-Fard, S.; Rezaei Nejad, A.; Sorkheh, K.; Sofo, A. Exogenous salicylic acid positively affects morpho-physiological and molecular responses of Impatiens walleriana plants grown under drought stress. Int. J. Environ. Sci. Technol. 2021, 19, 969–984.
- Lee, B.-R.; Islam, M.T.; Park, S.-H.; Jung, H.-i.; Bae, D.-W.; Kim, T.-H. Characterization of salicylic acid-mediated modulation of the drought stress responses: Reactive oxygen species, proline, and redox state in Brassica napus. Environ. Exp. Bot. 2019, 157, 1–10.
- Shemi, R.; Wang, R.; Gheith, E.-S.; Hussain, H.A.; Hussain, S.; Irfan, M.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress. Sci. Rep. 2021, 11, 3195.
- Ahmad, A.; Aslam, Z.; Naz, M.; Hussain, S.; Javed, T.; Aslam, S.; Raza, A.; Ali, H.M.; Siddiqui, M.H.; Salem, M.Z. Exogenous salicylic acid-induced drought stress tolerance in wheat (Triticum aestivum L.) grown under hydroponic culture. PLoS ONE 2021, 16, e0260556.
- Khalvandi, M.; Siosemardeh, A.; Roohi, E.; Keramati, S. Salicylic acid alleviated the effect of drought stress on photosynthetic characteristics and leaf protein pattern in winter wheat. Heliyon 2021, 7, e05908.
- Damalas, C.A. Improving drought tolerance in sweet basil (Ocimum basilicum) with salicylic acid. Sci. Hortic. 2019, 246, 360–365.
- Abbaszadeh, B.; Layeghhaghighi, M.; Azimi, R.; Hadi, N. Improving water use efficiency through drought stress and using salicylic acid for proper production of Rosmarinus officinalis L. Ind. Crops Prod. 2020, 144, 111893.
- Tiwari, A.; Kumar, P.; Singh, S.; Ansari, S. Carbonic anhydrase in relation to higher plants. Photosynthetica 2005, 43, 1–11.
- Hayat, S.; Hasan, S.A.; Fariduddin, Q.; Ahmad, A. Growth of tomato (Lycopersicon esculentum) in response to salicylic acid under water stress. J. Plant Interact. 2008, 3, 297–304.
- Tayyab, N.; Naz, R.; Yasmin, H.; Nosheen, A.; Keyani, R.; Sajjad, M.; Hassan, M.N.; Roberts, T.H. Combined seed and foliar pre-treatments with exogenous methyl jasmonate and salicylic acid mitigate drought-induced stress in maize. PLoS ONE 2020, 15, e0232269.
- Wedow, J.M.; Ainsworth, E.A.; Li, S. Plant biochemistry influences tropospheric ozone formation, destruction, deposition, and response. Trends Biochem. Sci. 2021, 46, 992–1002.
- Oksanen, E.; Pandey, V.; Pandey, A.; Keski-Saari, S.; Kontunen-Soppela, S.; Sharma, C. Impacts of increasing ozone on Indian plants. Environ. Pollut. 2013, 177, 189–200.
- Yalpani, N.; Enyedi, A.J.; León, J.; Raskin, I. Ultraviolet light and ozone stimulate accumulation of salicylic acid, pathogenesis-related proteins and virus resistance in tobacco. Planta 1994, 193, 372–376.
- Ogawa, D.; Nakajima, N.; Tamaoki, M.; Aono, M.; Kubo, A.; Kamada, H.; Saji, H. The isochorismate pathway is negatively regulated by salicylic acid signaling in O3-exposed Arabidopsis. Planta 2007, 226, 1277–1285.
- Pellegrini, E.; Trivellini, A.; Cotrozzi, L.; Vernieri, P.; Nali, C. Involvement of Phytohormones in Plant Responses to Ozone. In Plant Hormones under Challenging Environmental Factors; Springer: Berlin, Germany, 2016; pp. 215–245.
- Marchica, A.; Cotrozzi, L.; Lorenzini, G.; Nali, C.; Pellegrini, E. Antioxidants and Phytohormones Act in Coordination to Regulate Sage Response to Long Term Ozone Exposure. Plants 2022, 11, 904.
- Rao, M.V.; Davis, K.R. Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: The role of salicylic acid. Plant J. 1999, 17, 603–614.
- Xu, E.; Vaahtera, L.; Brosché, M. Roles of defense hormones in the regulation of ozone-induced changes in gene expression and cell death. Mol. Plant 2015, 8, 1776–1794.
- Kittipornkul, P.; Treesubsuntorn, C.; Thiravetyan, P. Effect of exogenous catechin and salicylic acid on rice productivity under ozone stress: The role of chlorophyll contents, lipid peroxidation, and antioxidant enzymes. Environ. Sci. Pollut. Res. 2020, 27, 25774–25784.
- Dubey, P.; Mishra, A.K.; Singh, A.K. Comparative analyses of genotoxicity, oxidative stress and antioxidative defence system under exposure of methyl parathion and hexaconazole in barley (Hordeum vulgare L.). Environ. Sci. Pollut. Res. 2015, 22, 19848–19859.
- Homayoonzadeh, M.; Hosseininaveh, V.; Haghighi, S.R.; Talebi, K.; Roessner, U.; Maali-Amiri, R. Evaluation of physiological and biochemical responses of pistachio plants (Pistacia vera L.) exposed to pesticides. Ecotoxicology 2021, 30, 1084–1097.
- Huang, Y.; Adeleye, A.S.; Zhao, L.; Minakova, A.S.; Anumol, T.; Keller, A.A. Antioxidant response of cucumber (Cucumis sativus) exposed to nano copper pesticide: Quantitative determination via LC-MS/MS. Food Chem. 2019, 270, 47–52.
- Homayoonzadeh, M.; Moeini, P.; Talebi, K.; Roessner, U.; Hosseininaveh, V. Antioxidant system status of cucumber plants under pesticides treatment. Acta Physiol. Plant. 2020, 42, 1–11.
- Fatma, F.; Kamal, A.; Srivastava, A. Exogenous application of salicylic acid mitigates the toxic effect of pesticides in Vigna radiata (L.) Wilczek. J. Plant Growth Regul. 2018, 37, 1185–1194.
- Nazish, T.; Huang, Y.-J.; Zhang, J.; Xia, J.-Q.; Alfatih, A.; Luo, C.; Cai, X.-T.; Xi, J.; Xu, P.; Xiang, C.-B. Understanding paraquat resistance mechanisms in Arabidopsis thaliana to facilitate developing paraquat-resistant crops. Plant Commun. 2022, 3, 100321.
- Kusumi, K.; Yaeno, T.; Kojo, K.; Hirayama, M.; Hirokawa, D.; Yara, A.; Iba, K. The role of salicylic acid in the glutathione-mediated protection against photooxidative stress in rice. Physiol. Plant. 2006, 128, 651–661.
- Meyer, P.; Van de Poel, B.; De Coninck, B. UV-B light and its application potential to reduce disease and pest incidence in crops. Hortic. Res. 2021, 8, 194.
- Mahdavian, K.; Ghorbanli, M.; Kalantari, K.M. Role of salicylic acid in regulating ultraviolet radiation-induced oxidative stress in pepper leaves. Russ. J. Plant Physiol. 2008, 55, 560–563.
- Escobar Bravo, R.; Chen, G.; Grosser, K.; Van Dam, N.M.; Leiss, K.A.; Klinkhamer, P.G. Ultraviolet radiation enhances salicylic acid-mediated defense signaling and resistance to Pseudomonas syringae DC3000 in a jasmonic acid-deficient tomato mutant. Plant Signal. Behav. 2019, 14, e1581560.
- Yüzbaşıoğlu, E.; Dalyan, E. Salicylic acid alleviates thiram toxicity by modulating antioxidant enzyme capacity and pesticide detoxification systems in the tomato (Solanum lycopersicum Mill.). Plant Physiol. Biochem. 2019, 135, 322–330.
- Liu, T.; Yuan, C.; Gao, Y.; Luo, J.; Yang, S.; Liu, S.; Zhang, R.; Zou, N. Exogenous salicylic acid mitigates the accumulation of some pesticides in cucumber seedlings under different cultivation methods. Ecotoxicol. Environ. Saf. 2020, 198, 110680.
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462.
- Santisree, P.; Jalli, L.C.L.; Bhatnagar-Mathur, P.; Sharma, K.K. Emerging roles of salicylic acid and jasmonates in plant abiotic stress responses. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress; Wiley: Hoboken, NJ, USA, 2020; pp. 342–373.
- Ahmad, F.; Singh, A.; Kamal, A. Salicylic Acid–Mediated Defense Mechanisms to Abiotic Stress Tolerance. In Plant Signaling Molecules; Woodhead Publishing: Sawston, UK, 2019; pp. 355–369.
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338.
- Rajendiran, K.; Ramanujam, M. Alleviation of ultraviolet-B radiation-induced growth inhibition of green gram by triadimefon. Biol. Plant. 2003, 46, 621–624.
- Del Valle, J.C.; Buide, M.L.; Whittall, J.B.; Valladares, F.; Narbona, E. UV radiation increases phenolic compound protection but decreases reproduction in Silene littorea. PLoS ONE 2020, 15, e0231611.
- Kosobryukhov, A.; Khudyakova, A.; Kreslavski, V. Impact of UV radiation on photosynthetic apparatus: Adaptive and damaging mechanisms. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I; Spinger: Berlin, Germany, 2020; pp. 555–576.
- Kovacs, E.; Keresztes, A. Effect of gamma and UV-B/C radiation on plant cells. Micron 2002, 33, 199–210.
- Hideg, É.; Jansen, M.A.; Strid, Å. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115.
- Singh, V.P.; Kumar, J.; Singh, M.; Singh, S.; Prasad, S.M.; Dwivedi, R.; Singh, M. Role of salicylic acid-seed priming in the regulation of chromium (VI) and UV-B toxicity in maize seedlings. Plant Growth Regul. 2016, 78, 79–91.
- Rao, M.V.; Paliyath, G.; Ormrod, D.P.; Murr, D.P.; Watkins, C.B. Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes (salicylic acid-mediated oxidative damage requires H2O2). Plant Physiol. 1997, 115, 137–149.
- Li, X.; Clarke, J.D.; Zhang, Y.; Dong, X. Activation of an EDS1-mediated R-gene pathway in the snc1 mutant leads to constitutive, NPR1-independent pathogen resistance. Mol. Plant-Microbe Interact. 2001, 14, 1131–1139.
- Petersen, M.; Brodersen, P.; Naested, H.; Andreasson, E.; Lindhart, U.; Johansen, B.; Nielsen, H.B.; Lacy, M.; Austin, M.J.; Parker, J.E. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell 2000, 103, 1111–1120.
- Han, Y.; Chaouch, S.; Mhamdi, A.; Queval, G.; Zechmann, B.; Noctor, G. Functional analysis of Arabidopsis mutants points to novel roles for glutathione in coupling H2O2 to activation of salicylic acid accumulation and signaling. Antioxid. Redox Signal. 2013, 18, 2106–2121.
- Freeman, J.L.; Garcia, D.; Kim, D.; Hopf, A.; Salt, D.E. Constitutively elevated salicylic acid signals glutathione-mediated nickel tolerance in Thlaspi nickel hyperaccumulators. Plant Physiol. 2005, 137, 1082–1091.
- Guan, C.; Ji, J.; Jia, C.; Guan, W.; Li, X.; Jin, C.; Wang, G. A GSHS-like gene from Lycium chinense maybe regulated by cadmium-induced endogenous salicylic acid and overexpression of this gene enhances tolerance to cadmium stress in Arabidopsis. Plant Cell Rep. 2015, 34, 871–884.
- Beyer, S.F.; Bel, P.S.; Flors, V.; Schultheiss, H.; Conrath, U.; Langenbach, C.J. Disclosure of salicylic acid and jasmonic acid-responsive genes provides a molecular tool for deciphering stress responses in soybean. Sci. Rep. 2021, 11, 20600.
- Chaouch, S.; Queval, G.; Vanderauwera, S.; Mhamdi, A.; Vandorpe, M.; Langlois-Meurinne, M.; Van Breusegem, F.; Saindrenan, P.; Noctor, G. Peroxisomal hydrogen peroxide is coupled to biotic defense responses by ISOCHORISMATE Synthase1 in a daylength-related manner. Plant Physiol. 2010, 153, 1692–1705.
- Chang, C.C.; Slesak, I.; Jordá, L.; Sotnikov, A.; Melzer, M.; Miszalski, Z.; Mullineaux, P.M.; Parker, J.E.; Karpinska, B.; Karpinski, S. Arabidopsis chloroplastic glutathione peroxidases play a role in cross talk between photooxidative stress and immune responses. Plant Physiol. 2009, 150, 670–683.
- Moons, A. Osgstu3 and osgtu4, encoding tau class glutathione S-transferases, are heavy metal-and hypoxic stress-induced and differentially salt stress-responsive in rice roots. FEBS Lett. 2003, 553, 427–432.
- Garretón, V.; Carpinelli, J.; Jordana, X.; Holuigue, L. The as-1 promoter element is an oxidative stress-responsive element and salicylic acid activates it via oxidative species. Plant Physiol. 2002, 130, 1516–1526.
- Sappl, P.G.; Oñate-Sánchez, L.; Singh, K.B.; Millar, A.H. Proteomic analysis of glutathione S-transferases of Arabidopsis thaliana reveals differential salicylic acid-induced expression of the plant-specific phi and tau classes. Plant Mol. Biol. 2004, 54, 205–219.
- Yasuda, M.; Ishikawa, A.; Jikumaru, Y.; Seki, M.; Umezawa, T.; Asami, T.; Maruyama-Nakashita, A.; Kudo, T.; Shinozaki, K.; Yoshida, S. Antagonistic interaction between systemic acquired resistance and the abscisic acid–mediated abiotic stress response in Arabidopsis. Plant Cell 2008, 20, 1678–1692.
- Park, S.-H.; Lee, B.-R.; Al Mamun, M.; Bae, D.-W.; Kim, T.-H. Characterization of salicylic acid-and abscisic acid-mediated photosynthesis, Ca2+ and H2O2 accumulation in two distinct phases of drought stress intensity in Brassica napus. Environ. Exp. Bot. 2021, 186, 104434.
- Liu, H.-T.; Liu, Y.-Y.; Pan, Q.-H.; Yang, H.-R.; Zhan, J.-C.; Huang, W.-D. Novel interrelationship between salicylic acid, abscisic acid, and PIP2-specific phospholipase C in heat acclimation-induced thermotolerance in pea leaves. J. Exp. Bot. 2006, 57, 3337–3347.
- Horváth, E.; Csiszár, J.; Gallé, Á.; Poór, P.; Szepesi, Á.; Tari, I. Hardening with salicylic acid induces concentration-dependent changes in abscisic acid biosynthesis of tomato under salt stress. J. Plant Physiol. 2015, 183, 54–63.
- Prodhan, M.Y.; Munemasa, S.; Nahar, M.N.-E.-N.; Nakamura, Y.; Murata, Y. Guard cell salicylic acid signaling is integrated into abscisic acid signaling via the Ca2+/CPK-dependent pathway. Plant Physiol. 2018, 178, 441–450.
- Nomura, H.; Komori, T.; Uemura, S.; Kanda, Y.; Shimotani, K.; Nakai, K.; Furuichi, T.; Takebayashi, K.; Sugimoto, T.; Sano, S. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat. Commun. 2012, 3, 926.
- Sun, T.; Huang, J.; Xu, Y.; Verma, V.; Jing, B.; Sun, Y.; Orduna, A.R.; Tian, H.; Huang, X.; Xia, S. Redundant CAMTA transcription factors negatively regulate the biosynthesis of salicylic acid and N-hydroxypipecolic acid by modulating the expression of SARD1 and CBP60g. Mol. Plant 2020, 13, 144–156.
- Kim, Y.; Gilmour, S.J.; Chao, L.; Park, S.; Thomashow, M.F. Arabidopsis CAMTA transcription factors regulate pipecolic acid biosynthesis and priming of immunity genes. Mol. Plant 2020, 13, 157–168.
- Wei, L.; Zhang, M.; Wei, S.; Zhang, J.; Wang, C.; Liao, W. Roles of nitric oxide in heavy metal stress in plants: Cross–talk with phytohormones and protein S-nitrosylation. Environ. Pollut. 2020, 259, 113943.
- Rai, K.K.; Rai, N.; Rai, S.P. Salicylic acid and nitric oxide alleviate high temperature induced oxidative damage in Lablab purpureus L plants by regulating bio-physical processes and DNA methylation. Plant Physiol. Biochem. 2018, 128, 72–88.
- Kotapati, K.V.; Palaka, B.K.; Ampasala, D.R. Alleviation of nickel toxicity in finger millet (Eleusine coracana L.) germinating seedlings by exogenous application of salicylic acid and nitric oxide. Crop J. 2017, 5, 240–250.
- Singh, A.P.; Dixit, G.; Kumar, A.; Mishra, S.; Kumar, N.; Dixit, S.; Singh, P.K.; Dwivedi, S.; Trivedi, P.K.; Pandey, V. A protective role for nitric oxide and salicylic acid for arsenite phytotoxicity in rice (Oryza sativa L.). Plant Physiol. Biochem. 2017, 115, 163–173.
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Corpas, F.J.; Ahmad, P. Salicylic acid-induced nitric oxide enhances arsenic toxicity tolerance in maize plants by upregulating the ascorbate-glutathione cycle and glyoxalase system. J. Hazard. Mater. 2020, 399, 123020.
- Wang, B.; Song, N.; Zhang, Q.; Wang, N.; Kang, Z. TaMAPK4 acts as a positive regulator in defense of wheat stripe-rust infection. Front. Plant Sci. 2018, 9, 152.
- Zhang, H.; Li, F.; Li, Z.; Cheng, J.; Chen, X.; Wang, Q.; Joosten, M.H.; Shan, W.; Du, Y. Potato StMPK7 is a downstream component of StMKK1 and promotes resistance to the oomycete pathogen Phytophthora infestans. Mol. Plant Pathol. 2021, 22, 644–657.
- Pan, J.; Guan, M.; Xu, P.; Chen, M.; Cao, Z. Salicylic acid reduces cadmium (Cd) accumulation in rice (Oryza sativa L.) by regulating root cell wall composition via nitric oxide signaling. Sci. Total Environ. 2021, 797, 149202.
- Agurla, S.; Sunitha, V.; Raghavendra, A.S. Methyl salicylate is the most effective natural salicylic acid ester to close stomata while raising reactive oxygen species and nitric oxide in Arabidopsis guard cells. Plant Physiol. Biochem. 2020, 157, 276–283.
