Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The main CGA in coffee is 5-O-caffeoylquinic acid (5-CQA). Coffee extracts are currently the most widely used source to enhance the antioxidant activity of foods. Due to the solubility of CGAs, their extraction is mainly performed with organic solvents. CGAs have been associated with health benefits, such as antioxidant, antiviral, antibacterial, anticancer, and anti-inflammatory activity, and others that reduce the risk of cardiovascular diseases, type 2 diabetes, and Alzheimer’s disease.
- chlorogenic acids
- coffee
- 5CQA
1. Introduction
The chlorogenic acids (CGAs) are a class of phenolic compounds widely distributed in various plants sources such as fruits, vegetables, coffee beans, tea, apples, and wine [1][2][3]. CGAs are esters of quinic acid (QA) and one trans-cinnamic acid residue such as caffeic acid (CA), p-coumaric acid (p-CoA), and ferulic acid (FA), which are known as caffeoylquinic acids (CQAs), p-coumaroylquinic acids (p-CoQAs) and feruloylquinic acid (FQAs) [1][2][3][4][5]. Caffeoylquinic acid may theoretically form four isomers, but only three are present in plants: 3-O-caffeoylquinic acid (3-CQA), neochlorogenic acid (5-O-caffeoylquinic acid, 5-CQA), or cryptochlorogenic acid (4-O-caffeoylquinic acid, 4-CQA). The most common isomer, 5-CQA, is an ester composed of caffeic acid and (−)-quinic acid and referred as chlorogenic acid [4][6]. According to the number of caffeoyl groups attached to the quinic acid, these CQAs can be classified into monophosphoylquinic acids (MCQAs), dicaffeoylquinic acids (DCQAs), tricaffeoylquinic acids (TCQAs), and tetracaffeoylquinic acids (tetra-CQAs) [3]. The chemical structures of the main CGAs are shown in Figure 1. The structural diversity and broad bioactivities of CGAs have increasingly attracted the attention of researchers [4][5][7][8][9][10]. It has been demonstrated that these compounds are potent antioxidants and may also exert other physiological activities. For example, there is evidence that CGAs possess a wide variety of bioactivities, such as antiparasitic [11], antibacterial [12], anti-inflammatory [13], neuroprotective [14], anticancer [15], antiglycemic [16], and antiviral [17]. In addition, it has been demonstrated that CGAs have therapeutic effects in the prevention and treatment of some chronic and cardiovascular diseases [5][18][19]. The following content aims to describe the main biological activities attributed to coffee CGAs, and their bioavailability and potential addition to different food matrices to obtain functional foods.
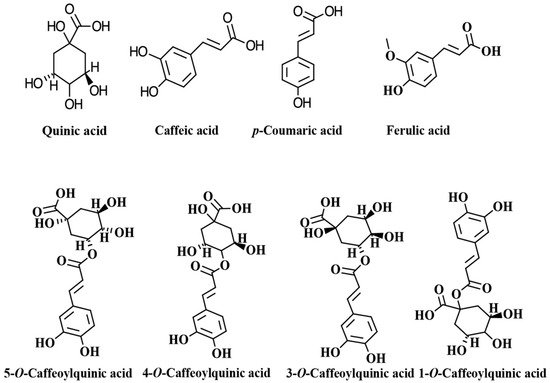
Figure 1. Chemical structures of main chlorogenic acids (CGAs) and isomers of caffeoylquinic acid.
2. Dietary Sources of Chlorogenic Acids (CGAs)
CGAs are a large family of esters of quinic acid and trans-cinnamic acids; up to date, at least 71 different chemical compounds are identified from different plants sources such as fruits, vegetables, coffee beans, tea, apples, artichoke, eggplant, and grapes [4][20][21]. However, those found in the highest concentration in plants are caffeoylquinic acids (CQAs), specifically mono- and di-CQAs, as well as the different isomeric forms of feruloylquinic acids (FQAs) [21][22]. Meinhart et al. [23] analyzed the CGAs concentration (CA, 3-CQA, 4-CQA, 5-CQA, 3,4-DQA, 3,5-DQA and 4,5-DQA) of 100 plants commonly used in Brazil as infusions. In their study, the highest concentrations of CGAS were yerba mate (Ilex paraguariensis), white and green tea (Camellia sinensis), and winter’s bark (Drimys winteri). A study of the presence of CGAs in 53 vegetables consumed in Southern Brazil reported the highest concentrations of 3-CQA, 5-CQA, and 4-CQA in collard greens and chicory whereas the highest concentration of 3,4-DQA, 3,5-DQA, and 4,5-DQA were found in bay leaves and mustard [20]. At present, green coffee beans and yerba mate are recognized as the most important plant sources of CGAs, accounting for up to 6 to 12% in the case of green coffee and 9% for mate. 5-CQA is the most abundant CGA in green coffee beans, with a concentration of about 100 mg/g (dry basis), representing 76 to 84% of the total content of CGAs [4]. The main food sources of CGAs are shown in Table 1.
Table 1. Main sources of CGAs.
| Source | Concentration (g/100 g) 1 (dm) |
CGA Composition | References |
|---|---|---|---|
| Artichoke | 1–8 | 5-CQA, 1,5-DCQA 3,4-DCQA and DCQA |
[24] |
| Artichoke leaves | 0.92 | CA, 3-CQA, 4-CQA, 5-CQA, 3,4-DQA, 3,5-DQA and 4,5-DQA | [23] |
| Sweet potato leaves | - | 3-CQA, 3,4-DCQA, 3,5-DCQA, 4,5-DCQA and 3,4,5-TCQA |
[15] |
| White tea (Camelia sinensis) leaves | 1.64 | 3-CQA, 4-CQA, 5-CQA, 3,4-DQA, 3,5-DQA and 4,5-DQA | [23] |
| Green tea (Camelia sinensis) leaves | 1.32 | 3-CQA, 4-CQA, 5-CQA, 3,4-DQA, 3,5-DQA and 4,5-DQA | [23] |
| Yerba mate (Ilex paraguariensis) leaves and thalli | 9.19 | 3-CQA, 4-CQA, 5-CQA, 3,4-DQA, 3,5-DQA and 4,5-DQA | [23] |
| Green coffee beans | 4.10–11.3 2 | CQA, FQA and DCQA | [25] |
| Apples | 0.38 0–0.2 g/L (juice) |
3-CQA, 5-CQA, 4,5-DCQA | [26] |
| Pears | 0.28 0–0.24 g/L (juice) |
3-CQA, 5-CQA, 3,6-DCQA | [27] |
| Blueberries | 2 | 5-CQA, 3-FQA | [28] |
| Grapes | 0.15 | 5-CQA, CoQA | [29] |
| Spinach | 0.2 | p-CoQA | [30] |
| Beans and peas | 0.12 | p-CoQA | [31] |
| Stone fruits | 0.01–0.6 | p-CoQA, 5-CQA, FQA, 4,5-DCQA, 3,4-TCQA | [32] |
| Potato tubers | 0.5–1.2 | CQA; DCQA | [33] |
1 Units may have been changed for consistency and expressed in dry matter (dm). 2 It depends on the variety and geographic origin of the coffee.
Coffee as a Source of CGAs
Coffee is one of the most widely consumed beverages in the world. This infusion contains several compounds that can exert beneficial biological activities for human health. Many beneficial effects have been investigated, mainly attributed to caffeine and other substances, such as polyphenols, mainly chlorogenic acids [1][34]. There are at least 30 different types of CGAs found in coffee, and this includes caffeoylquinic acids (CQAs), dicaffeoylquinic acids (DCQAs), tricaffeoylquinic acids (TCQAs), feruloylquinic acids (FQAs), and p-coumaroylquinic acids (p-CoQAs) [35]. One cup (200 mL) of coffee brew contains between 20–675 mg of CGAs depending on the variety of coffee and brewing method [36].
Whether green or roasted, the beneficial health effects of coffee have been attributed to the high content of CGAs and the antioxidant activity provided by the phenolic compounds in green coffee in addition to the those produced during the roasting process [37]. The concentration of active polyphenols inside green coffee depends on the variety of the bean and its geographical origin; in beverages, it also depends on the brewing process [38]. During the coffee roasting process, phenolic compounds undergo a series of intermolecular and intramolecular reactions and interactions [21]. Higher concentration of CGAs in lightly roasted coffee over dark roasted coffee has been established; however, the highest concentration of CGAs is found in green coffee beans [39]. In green coffee, CQAs alone account for up to 80% of the total CGAs and among CQAs, 5-CQA account for almost 60%. Thus, 5-CQA is the most studied isomer of the CGAs and is responsible for the bitter and astringent taste in coffee [25].
3. Extraction of Chlorogenic Acids (CGAs) from Coffee
The extraction recovery of a wide variety of compounds from vegetal species is a critical step in the production of bioactive substances. The chemical properties of CGAs such as thermal stability, solubility, and oxidation-reduction reactions, need to be considered when combined with other substances [21]. Studies focused on the development of new extraction methods of CGA’s have been made over the last couple of decades, mainly focusing on increasing mass transfer and extraction yields while minimizing the use of toxic organic solvents and energy consumption [40][41][42][43][44][45][46].
3.1. Organic Solvent Extraction
Madhava-Naidu et al. [46] extracted green coffee CGAs by sterilizing the beans at 120 °C for 20 min and Soxhlet extraction with hexane at different rates. The samples were then separated in glass columns and extracted with selected solvents at different ratios (80:20, 70:30, and 60:40) using mixtures of isopropanol and water. They obtained the best extraction yield (29.1%) and a CGAs content of 29.7% when they used a 60:40 isopropanol-water ratio in Robusta coffee, whereas in Arabica coffee the yield of extraction and CGAs content were 27.3 and 30.2%, respectively. Suárez-Quiroz et al. [40] compared four different methods of CGAs extraction using different solvents (water, aqueous methanol, aqueous isopropanol, and ethyl acetate). The extract yield values were not significantly different, demonstrating the high solubility of CGAs in organic solvents. CGA’s extraction from green coffee (C. arabica) using water at 80 °C and activated carbon were not significantly different from the values of the previous investigation [47]. Thus, activated carbon is a suitable and more eco-friendly extraction method with a minimum of 97% CGAs purity in the extract was reported in this study [47].
Dibert et al. [48] tested the effect of different physicochemical parameters (temperature, particle diameter, and solvent-mass ratio) when extracting CGAs from green coffee beans with a methanol-water extraction (70:30 ratio) at three different temperatures (30, 40, and 50 °C). The highest yield of extraction of CGAs (18.1%) was obtained at 40 and 50 °C, when a mass-solvent ratio of 1:4 w/v was used. By increasing the mass ratio of green coffee beans an improvement in the yield of extraction of CGAs can be achieved.
3.2. Pulsed Electric Field Extraction
Bilge et al. [45] evaluated the effect of pulsed electric field on green and roasted C. arabica beans as a pretreatment by exposing them to monopolar pulses of 2 Hz with an interval of 0.5 s and generating an electric field of 28 kV/10 cm with water at 20 °C. The use of an electric field increased radical scavenging activity up to 31% and 11%, for green and roasted coffee beans, respectively, compared to untreated samples confirming that using electric pulses as a pretreatment before extraction can enhance the phenolic content extraction and reduce Maillard reaction products that occur at high temperatures of extraction and during the coffee roasting process. Phongsupa et al. [44] studied the extraction of CGAs by pulse electric field induction over C. arabica. The number of pulses and concentrations for this study was set to 1000 pulses at 5 kV and 62.7% methanol-water solution as solvent. The mass-solvent ratio with the most effective extraction was 0.75 g/mL and 30 s of blending which had the CGAs content of 9.8 μg/g. However, the results obtained in this study showed that an increase in the sample-solvent ratio leads to a higher concentration of CGAs [45].
4. Bioavailability of CGAs
Numerous studies have shown the potential health benefits of CGAs. Consequently, evidence of the absorption and bioavailability of CGAs is needed to evaluate these compounds’ health benefits fully. However, the absorption and bioavailability of CGAs are controversial due to the significant interindividual differences regarding their utilization, metabolism, and excretion found in scientific and clinical studies.
4.1. Absorption of CGAs
Past studies have considered that 5-CQA, such as other phenolic compounds, could be poorly absorbed by the digestive system [49]. However, other studies have shown that a part of this compound can be absorbed intact in the stomach and/or small intestine [50][51]. It is now known that, on average, almost one third of the 5-CQA obtained from the diet is absorbed from the gastrointestinal tract into the bloodstream, although its absorption varies among humans [49][52][53][54][55]. For example, after coffee consumption, two plasma concentration peaks of CGAs corresponding to 5-CQA and DCQA were found at 0.5 to 1.0 and 1.5 to 4.0 h, respectively [50]. Furthermore, Mubarak et al. [56] reported a higher concentration of intact 5-CQA in plasma at 2.5 h in all healthy volunteers following intake of pure 5-CQA (400 mg, approximately corresponding to two cups of coffee). Therefore, it has been suggested that 5-CQA is absorbed through at least two pathways. One pathway may involve immediate absorption of intact 5-CQA in the stomach and/or upper gastrointestinal tract, whereas the other involves slow absorption of intact 5-CQA throughout the small intestine [4]. Additionally, Erk et al. [57] reported that a high intake of 5-CQA from coffee could modify gastrointestinal transport and influence its absorption and metabolism.
4.2. Metabolization of CGAs
The human metabolism of CGAs is somewhat complex but well defined. The main pathways of CGAs metabolism are as follows: (i) absorbed non-transformed, (ii) absorbed in the stomach or small intestine, with or without hydrolysis, and then conjugated (sulfate, glucuronide, or methyl) or otherwise metabolized (hydrogenated, α- or β-oxidized, conjugated with glycine), (iii) undergo gut microbiota-mediated metabolism, after which the microbial catabolites are absorbed without further change, or (iv) undergo metabolism via the intestinal microbiota, after which the microbial catabolites are absorbed and undergo mammalian phase II metabolism (conjugation with glucuronide, sulfate, methyl, or glycine) or are otherwise metabolized (hydrogenated, dehydrogenated, α- or β-oxidations) [58]. Thus, it has been found that 33% of the total intake of 5-CQA from the diet is absorbed intact, unhydrolyzed, in the stomach or upper intestine and subsequently passes into the bloodstream. About 7% of the total intake of 5-CQA is absorbed through the small intestine by hydrolysis to CA and QA. Furthermore, part of the metabolism of 5-CQA is mediated by the colonic microbiota. In some studies, traces of 5-CQA have been found in the urine (0.3–2.3%) after ingesting foods with a high content of this phenolic compound, indicating that the absorption of intact 5-CQA is intensively metabolized [50][52]. It is important to highlight that the absorbed part of 5-CQA and its metabolites can induce various physiological effects through the bloodstream, while unabsorbed 5-CQA can induce biological effects throughout the digestive tract, such as modification of the gut microbiota [52][59].
5. Incorporation of CGAs into Food Matrices
The growing demand for healthier foods and better lifestyles is relevant for consumers nowadays. Nutrition scientists and food scientists have established that the best way to enrich and fortify food products in overall nutrient intake with minimum side effects is by using extracts and compounds from natural sources (cereals, greens, fruits, etc.) [60]. Chlorogenic acids obtained from distinct vegetal species and their wastes can be used as natural ingredients for different food products. Plant foods such as vegetables and fruits are the main source of calories, carbohydrates, and other essential compounds for the human body and play an important role in human health, such as polyphenols [61]. To increase the intake of polyphenols and the level of acceptance among consumers around the globe, studies on food technology using polyphenols have been increasing over the last couple of decades. Polyphenols are a very significant source of phytochemicals which have been used by the pharmaceutical industry for many years in a wide variety of products [62].
Corso et al. [63] studied the antioxidant properties of CGAs and enriching coffee itself. CGAs extract was obtained by a series of percolation stages with pressure water at 180 °C in the first and 100 °C at the final stages. The extract was freeze-dried and added to instant coffee formulations. It was added to obtain a concentration of 7% polyphenols in four different instant coffee formulations. The green coffee extract had 14% CQAs, and 5-CQA was the most abundant [25]. The formulation of instant coffee increased its 5-CQA content and showed 3.18 g/100 g compared to the control 1.20 g/100 g. Moreover, CGAs addition increases antioxidant activity in the instant coffee (evaluated by the ABTS and Folin methods); for enriched coffee, the antioxidant activity was in a range of 30.9–32.0 g of Trolox/100 g, whereas for the control, the content was 24.0–25.6 g of Trolox/100 g. Additionally, the authors reported that the antioxidant activity is not significantly affected by the roasting process. Since the polyphenols reduction (CGAs) is balanced by increasing melanoidin content, Vignoli et al. [64] had similar results. Moreover, no significant difference was found when the sensory evaluation was performed, and all formulations were accepted, obtaining average scores from 6.6 to 7.7 on a hedonic 10-point scale.
Bakery products are well-known sources of energy and nutrients such as carbohydrates, proteins, minerals, and vitamins. However, these also lack antioxidant-rich polyphenolic compounds, fiber, minerals, vitamin B6, thiamine, folate, vitamin E, and some phytochemicals, mostly because the bakery products are formulated with refined wheat flour [65][66]. A study to determine the functional and technological properties of GCE rich in hydroxycinnamic acids (CGAs) in wheat flour and bread was made by Mukkundur et al. [67]. Three levels of GCE obtained from defatted and decaffeinated C. canephora green coffee beans were added at 1, 1.5, and 2% on wheat flour. A decrease in total polyphenols (TTP), CGAs, and radical scavenging activity (RSA), 20.0, 36.2, and 93.1%, respectively, was observed due to the high temperature of extraction (80 °C). While the extract obtained at 60 °C was higher for TTP, CGAs, and RSA (21.4, 37.3, and 94.4%, respectively) due to the thermal sensibility of polyphenols [41]. In bread, CGAs addition was found to improve overall key parameters. First, the addition of 2% GCE improved the bread volume (565 cc) compared to the control (525 cc) due to polyphenols’ interaction with gluten proteins and starch giving more tenacity and extensibility to the dough. GCE addition increased the greenness of bread crumb and reduced yellowness and lightness. This effect was expected because the green color indicates CGAs hydrolyzation and thermal degradation [41]. The texture of the bread was not significantly affected by GCE addition; however, the bread containing 2% GCE was softer (4.38 N) compared to the control (4.81 N). The content of TPP, RSA, and CGAs in bread significantly increased in all treatments; for TPP, the content was 0.16, 0.25, and 0.34% (for 1, 1.5, and 2% GCE, respectively) compared to the control (0.02%). RSA content increased from 12.7% for the control to 68.5% at the highest level of GCE addition. CGAs content increased from 0.28 to 0.54% with the treatments, whereas CGAs were not detected in control. Finally, for the sensory evaluation, the authors reported that despite the benefits of GCE addition in all three levels, the maximum level of enrichment without affecting the overall quality of the bread (especially taste) was 1.5% GCE.
The influence of addition of green coffee extract (GCE) prepared in aqueous extraction at 110 °C for 15 min with high content of chlorogenic acids in fried doughnuts was analyzed [68]. The GCE had a content of 25.5 g/100 g of polyphenols. The most abundant polyphenol was 5-CQA, two isomers of 5-CQA hydrolyzation (3-CQA and 4-CQA), and another ferulic compound (5-FQA). Results indicated a significant increase of antioxidant activity up to 37, 45, and 50% using three addition levels (0.25, 0.50, and 1%, respectively) compared to the control. The authors compared this enhancement with another extract from a different source of chlorogenic acids (green tea extract) GTE, and they found that GTE addition increased antioxidant activity by 22, 28, and 29% compared to the control. These data confirm that green coffee remains the vegetal species with the highest concentration of polyphenols, for example, CGAs. However, it is relevant to consider that the frying process reduces the 5-CQA concentration at the final by hydrolyzing the diesters to monoesters.
Moreover, the addition of raw coffee beans in food products has been attempted. Another study on wheat bread properties was developed by Zain et al. [69]. The authors proposed to use grounded green coffee beans (GCB) instead of an extract to substitute the flour at three levels of addition (3, 5, and 7% GCB). After baking, there was a significant increase in the TPP content since the addition of 3, 5, and 7% GCB increased TPP in bread up to eight times at the highest level (1.61 mg GAE/g) compared to control (0.26 mg GAE/g) and even at the first level (3% GCB) TPP content was nearly twice as much compared to whole wheat bread. Since polyphenols were detected in control wheat bread, the authors concluded that it was the ferulic acid present naturally in wheat flour after the milling process and amino acids and smaller peptides formed in proteolysis of protein wheat flour during fermentation of bread. Antioxidant properties of the bread were also analyzed, and they found that with GCB addition, the RSA increased significantly (up to three times more) than the control. As explained by several authors, the increase in antioxidant activity is mainly attributed to the presence of phytochemicals in GCB (mainly chlorogenic acids). However, GCB addition did affect the sensory properties of bread. According to the sensory evaluation (shape, texture, attractiveness, color, chewability, odor, and taste) for control, the overall score was around 7.3–7.5, whereas for 3, 5, and 7% GCB bread, the scores were significantly lower (6.3, 5, and 4.2, respectively). Color and volume of bread were negatively affected; the more GCB was added, the color of the crumb and crust turned green and reduced volume. This suggested that CGB addition probably affects the stability of the gluten matrix, making a compact structure. It is well known that bread types depend on cultural and geographic requirements. Therefore, enriched food formulation must be designed according to the target market [70].
The fortification of dairy products has increased in the last couple of decades [71]. A study on the field of fortification of dairy products using encapsulated green coffee extract was made by Rahpeyma et al. [72]. The extract was obtained with boiling water at 110 °C for 30 min, cooled at room temperature and filtered. The authors used an emulsion microencapsulation technique using glycerol monostearate (GMS) and canola oil, and shaking at 4000 rpm and 70 °C. They used three levels of GCE addition (0.25, 0.5, and 1%) and three levels of encapsulated green coffee extract (EGCE) at 1.25, 2.5, and 5% EGCE. The TPP content and RSA of the extract were found to be 39 and 74%, respectively. Under the microencapsulation condition, the coating material greatly impacted the retention of phenolic compounds and antioxidant activity of the extract when added to kashk. The enrichment of kashk does not affect rheological properties such as viscosity across all treatments. Noteworthily, acidification by GCE, EGCE, and lactic fermentation caused a decrease in the negative electric charge of the micelles by degrading the calcium and inorganic phosphates.
Another study on dairy products was developed by Dönmez et al. [73]. They added green coffee powder (GCP) at two levels (1 and 2%) into homemade yogurt to analyze the polyphenols´ activity and interaction with proteins from yogurt. The coffee addition reduced the serum release rate (syneresis) in yogurt. The serum separation was significantly restricted by half of the control rate with the highest addition level of GCP. Polyphenols are highly reactive to proteins since they can form protein-polyphenol complexes through multiple weak interactions (mainly hydrophobic, van der Walls, and hydrogen bridge-binding) formed between protein side chains and polyphenol aromatic rings [74]. However, interactions between GCP and proteins were strengthening the gel structure of yogurt and hence, affected its rheological behavior. The yogurt consistency was increased during the first 14 days of storage. There was no significant change in flow index of yogurt with the highest level of GCP during 21 days of storage. Color significantly changed with GCP addition; green color increased over storage time up to 40% of the greenness for day 21.
In dairy-free milk-based products, the effect of CGA addition was investigated by Seczyk et al. [75] with green coffee phenolics (CGAs) added to soymilk. The extract was obtained by aqueous heat-assisted extraction using 10 g of green coffee, boiled in 100 mL of water at 110 °C, allowed to cool down at room temperature for 1 h with continuous orbital shaking and then filtered (Whatman No. 4). Six levels of addition were used in the study (0.0025, 0.05, 0.1, 0.25, 0.5, and 1 mg of phenolics (GAE, gallic acid equivalent) per 1 mL). Compared to the control, the content of phenolics increased up to 70% at the highest level of addition of CGAs. Antioxidant activity and reducing power were significantly affected, increasing the content up to 3.5–13.8 times more than the control.
Furthermore, CGAs addition also improved the digestibility of starch and proteins in soymilk, increasing digestibility up to 17.9% higher than the unfortified soymilk. CGE addition improved soymilk aroma and texture, with grass-lemon notes. The taste was positively affected by CGAs addition showing a score range between 5–5.3 whereas the control had a 4.7 score; however, at the highest level of fortification, the taste score significantly decreased (3.0) [75].
This entry is adapted from the peer-reviewed paper 10.3390/molecules27113400
References
- Bułdak, R.J.; Hejmo, T.; Osowski, M.; Bułdak, Ł.; Kukla, M.; Polaniak, R.; Birkner, E. The impact of coffee and its selected bioactive compounds on the development and progression of colorectal cancer in vivo and in vitro. Molecules 2018, 23, 3309.
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358.
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current advances in naturally occurring caffeoylquinic acids: Structure, bioactivity, and synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516.
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158.
- Li, L.; Su, C.; Chen, X.; Wang, Q.; Jiao, W.; Luo, H.; Tang, J.; Wang, W.; Li, S.; Guo, S. Chlorogenic acids in cardiovascular disease: A review of dietary consumption, pharmacology, and pharmacokinetics. J. Agric. Food Chem. 2020, 68, 6464–6484.
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013, 38, 413–419.
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 Diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 165–170.
- Wang, L.N.; Wang, W.; Hattori, M.; Daneshtalab, M.; Ma, C.M. Synthesis, Anti-HCV, antioxidant and reduction of intracellular reactive oxygen species generation of a chlorogenic acid analogue with an amide bond replacing the ester bond. Molecules 2016, 21, 737.
- Ye, X.; Li, J.; Gao, Z.; Wang, D.; Wang, H.; Wu, J. Chlorogenic acid inhibits lipid deposition by regulating the enterohepatic FXR-FGF15 pathway. BioMed Res. Int. 2022, 2022, 4919153.
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74.
- Smith, P.; Kiong Ho, C.; Takagi, Y.; Djaballah, H.; Shuman, S. Nanomolar inhibitors of Trypanosoma brucei RNA triphosphatase. MBio 2016, 7, e00058-16.
- Zhu, X.; Zhang, H.; Lo, R. Phenolic compounds from the leaf extract of artichoke (Cynara scolymus L.) and their antimicrobial activities. J. Agric. Food Chem. 2004, 52, 7272–7278.
- Zhao, Z.; Hee, S.S.; Satsu, H.; Totsuka, M.; Shimizu, M. 5-Caffeoylquinic acid and caffeic acid down-regulate the oxidative stress- and TNF-α-induced secretion of Interleukin-8 from Caco-2 Cells. J. Agric. Food Chem. 2008, 56, 3863–3868.
- Tian, X.; An, L.; Gao, L.Y.; Bai, J.P.; Wang, J.; Meng, W.H.; Ren, T.S.; Zhao, Q.C. Compound MQA, a caffeoylquinic acid derivative, protects against NMDA-induced neurotoxicity and potential mechanisms in vitro. CNS Neurosci. Ther. 2015, 21, 575–584.
- Kurata, R.; Adachi, M.; Yamakawa, O.; Yoshimoto, M. Growth suppression of human cancer cells by polyphenolics from sweetpotato (Ipomoea batatas L.) leaves. J. Agric. Food Chem. 2006, 55, 185–190.
- Pramanik, K.C.; Bhattacharya, P.; Biswas, R.; Bandyopadhyay, D.; Mishra, M.; Chatterjee, T.K. Hypoglycemic and antihyperglycemic activity of leaf extract of Pluchea indica Less. Orient. Pharm. Exp. Med. 2006, 6, 232–236.
- Mahmood, N.; Moore, P.S.; De Tommasi, N.; De Simone, F.; Colman, S.; Hay, A.J.; Pizza, C. Inhibition of HIV infection by caffeoylquinic acid derivatives. Antivir. Chem. Chemother. 2016, 4, 235–240.
- Hung, T.M.; Na, M.K.; Thuong, P.T.; Su, N.D.; Sok, D.E.; Song, K.S.; Seong, Y.H.; Bae, K.H. Antioxidant activity of Caffeoyl quinic acid derivatives from the roots of Dipsacus asper wall. J. Ethnopharmacol. 2006, 108, 188–192.
- El-Seedi, H.R.; El-Said, A.M.A.; Khalifa, S.A.M.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.K.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of Hydroxycinnamic Acids. J. Agric. Food Chem. 2012, 60, 10877–10895.
- Meinhart, A.D.; Damin, F.M.; Caldeirão, L.; de Jesus Filho, M.; da Silva, L.C.; da Silva Constant, L.; Teixeira Filho, J.; Wagner, R.; Teixeira Godoy, H. Study of new sources of six chlorogenic acids and caffeic acid. J. Food Compos. Anal. 2019, 82, 103244.
- Upadhyay, R.; Mohan Rao, L.J. An outlook on chlorogenic acids—Occurrence, chemistry, technology, and biological activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984.
- Frosi, I.; Montagna, I.; Colombo, R.; Milanese, C.; Papetti, A. Recovery of chlorogenic acids from agri-food wastes: Updates on green extraction techniques. Molecules 2021, 26, 4515.
- Meinhart, A.D.; Damin, F.M.; Caldeirão, L.; da Silveira, T.F.F.; Filho, J.T.; Godoy, H.T. Chlorogenic acid isomer contents in 100 plants commercialized in Brazil. Food Res. Int. 2017, 99, 522–530.
- Lattanzio, V.; Cicco, N.; Linsalata, V. Antioxidant activities of artichoke phenolics. Acta Hortic. 2005, 681, 421–428.
- Farah, A.; Donangelo, C.M. Phenolic compounds in Coffee. Braz. J. Plant Physiol. 2006, 18, 23–36.
- Lu, Y.; Foo, L.Y. Identification and quantification of major polyphenols in apple pomace. Food Chem. 1997, 59, 187–194.
- Wald, B.; Wray, V.; Galensa, R.; Herrmann, K. Malonated flavonol glycosides and 3,5-dicaffeoylquinic acid from pears. Phytochemistry 1989, 28, 663–664.
- Rodriguez-Mateos, A.; Cifuentes-Gomez, T.; Tabatabaee, S.; Lecras, C.; Spencer, J.P.E. Procyanidin, anthocyanin, and chlorogenic acid contents of highbush and lowbush blueberries. J. Agric. Food Chem. 2012, 6, 5772–5778.
- Somers, T.C.; Vérette, E.; Pocock, K.F. Hydroxycinnamate esters of Vitis vinifera: Changes during white vinification, and effects of exogenous enzymic hydrolysis. J. Sci. Food Agric. 1987, 40, 67–78.
- Mattila, P.; Hellström, J. Phenolic acids in potatoes, vegetables, and some of their products. J. Food Compos. Anal. 2007, 20, 152–160.
- Strack, D.; Hartfeld, F.; Austenfeld, F.A.; Grotjahn, L.; Wray, V. Coumaroyl-, caffeoyl- and feruloyltartronates and their accumulation in Mung bean. Phytochemistry 1985, 24, 147–150.
- Donovan, J.L.; Meyer, A.S.; Waterhouse, A.L. Phenolic composition and antioxidant activity of prunes and prune juice (Prunus domestica). J. Agric. Food Chem. 1998, 46, 1247–1252.
- Malmberg, A.G.; Theander, O. Determination of chlorogenic acid in potato tubers. J. Agric. Food Chem. 1985, 33, 549–551.
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244.
- Franca, A.S.; Oliveira, L.S. Coffee and its by-products as sources of bioactive compounds. In Coffee: Production, Consumption and Health Benefits, 1st ed.; Massey, J.L., Ed.; Nova Science Publishers: New York, NY, USA, 2016; pp. 1–28.
- Jeszka-Skowron, M.; Sentkowska, A.; Pyrzyńska, K.; de Peña, M.P. Chlorogenic acids, caffeine content and antioxidant properties of green coffee extracts: Influence of green coffee bean preparation. Eur. Food Res. Technol. 2016, 242, 1403–1409.
- Perrone, D.; Farah, A.; Donangelo, C.M.; de Paulis, T.; Martin, P.R. Comprehensive analysis of major and minor chlorogenic acids and lactones in economically relevant brazilian coffee cultivars. Food Chem. 2008, 106, 859–867.
- Silva, C.T.; de Souza, M.C.; Machado, A.P.D.F.; do Nascimento, R.D.P.; da Cunha, D.T.; Bezerra, R.M.N.; Rostagno, M.A. Thermal stability and sensory evaluation of a bioactive extract from roasted coffee (Coffea arabica) beans added at increasing concentrations to conventional bread. J. Food Process. Preserv. 2021, 45, e15955.
- Somporn, C.; Kamtuo, A.; Theerakulpisut, P.; Siriamornpun, S. Effects of roasting degree on radical scavenging activity, phenolics and volatile compounds of arabica coffee beans (Coffea arabica L. cv. Catimor). Int. J. Food Sci. Technol. 2011, 46, 2287–2296.
- Suárez-Quiroz, M.; Alonso Campos, A.; Valerio Alfaro, G.; González-Ríos, O.; Villeneuve, P.; Figueroa-Espinoza, M. Isolation of green coffee chlorogenic acids using activated carbon. J. Food Compos. Anal. 2014, 33, 55–58.
- Upadhyay, R.; Ramalakshmi, K.; Jagan Mohan Rao, L. Microwave-assisted extraction of chlorogenic acids from green coffee beans. Food Chem. 2012, 130, 184–188.
- Yang, Z.; Tan, Z.; Li, F.; Li, X. An effective method for the extraction and purification of chlorogenic acid from ramie (Boehmeria nivea L.) leaves using acidic ionic liquids. Ind. Crops Prod. 2016, 89, 78–86.
- Budryn, G.; Nebesny, E.; Podsȩdek, A.; Zyzelewicz, D.; Materska, M.; Jankowski, S.; Janda, B. Effect of different extraction methods on the recovery of chlorogenic acids, caffeine and Maillard reaction products in coffee beans. Eur. Food Res. Technol. 2009, 228, 913–922.
- Phongsupa, J.; Yawootti, A.; Wattanutchariya, W. Chlorogenic acid extraction of local coffee beans by pulsed electric field. AIP Conf. Proc. 2021, 2397, 020004.
- Bilge, G.; Yurdakul, M.; Buzrul, S.; Bulut, O. Evaluation of the effect of pulsed electric field on coffee arabica beans. Food Bioproc. Technol. 2022, 15, 1073–1081.
- Madhava Naidu, M.; Sulochanamma, G.; Sampathu, S.R.; Srinivas, P. Studies on extraction and antioxidant potential of green coffee. Food Chem. 2008, 107, 377–384.
- Rakotomalala, J.J. Diversité Biochimique des Caféiers: Analyse des Acides Hydroxycinnamiques, Bases Puriques et Diterpènes Glycosidiques. Particularités des Caféiers Sauvages de la Région Malgache (Mascarocoffea chev.), 1993. Ph.D. Thesis, Universite Montpellier II, Montpellier, France, 1992.
- Dibert, K.; Cros, E.; Andrieu, J. Solvent extraction of oil and chlorogenic acid from green coffee. Part II: Kinetic data. J. Food Eng. 1989, 10, 199–214.
- Farah, A.; de Paula Lima, J. Chlorogenic acids: Daily consumption through coffee, metabolism and potential health effects. In Coffee: Consumption and Health Implications, 1st ed.; Farah, A., Ed.; Royal Society of Chemistry: London, UK, 2019; pp. 364–415.
- Monteiro, M.; Farah, A.; Perrone, D.; Trugo, L.C.; Donangelo, C. Chlorogenic acid compounds from coffee are differentially absorbed and metabolized in humans. J. Nutr. 2007, 137, 2196–2201.
- Lafay, S.; Gil-Izquierdo, A.; Manach, C.; Morand, C.; Besson, C.; Scalbert, A. Chlorogenic acid is absorbed in its intact form in the stomach of rats. J. Nutr. 2006, 136, 1192–1197.
- Olthof, M.R.; Hollman, P.C.H.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71.
- Farah, A.; Duarte, G. Bioavailability and metabolism of chlorogenic acids from coffee. In Coffee in Health and Disease Prevention, 1st ed.; Preedy, V.R., Ed.; Academic Press: Oxford, UK, 2015; pp. 789–801.
- Nabavi, S.F.; Tejada, S.; Setzer, W.N.; Gortzi, O.; Sureda, A.; Braidy, N.; Daglia, M.; Manayi, A.; Nabavi, S.M. Chlorogenic acid and mental diseases: From chemistry to medicine. Curr. Neuropharmacol. 2017, 15, 471.
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J. Nutr. 2008, 138, 2309–2315.
- Mubarak, A.; Bondonno, C.P.; Liu, A.H.; Considine, M.J.; Rich, L.; Mas, E.; Croft, K.D.; Hodgson, J.M. Acute effects of chlorogenic acid on nitric oxide status, endothelial function, and blood pressure in healthy volunteers: A randomized trial. J. Agric. Food Chem. 2012, 60, 9130–9136.
- Erk, T.; Williamson, G.; Renouf, M.; Marmet, C.; Steiling, H.; Dionisi, F.; Barron, D.; Melcher, R.; Richling, E. Dose-dependent absorption of chlorogenic acids in the small intestine assessed by coffee consumption in ileostomists. Mol. Nutr. Food Res. 2012, 56, 1488–1500.
- Clifford, M.N.; Kerimi, A.; Williamson, G. Bioavailability and metabolism of chlorogenic acids (Acyl-quinic acids) in humans. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1299–1352.
- Mills, C.E.; Tzounis, X.; Oruna-Concha, M.J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P.E. In vitro colonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. Br. J. Nutr. 2015, 113, 1220–1227.
- Vicentini, A.; Liberatore, L.; Mastrocola, D. Functional foods: Trends and development. Ital. J. Food Sci. 2016, 28, 338–352.
- Yang, C.S.; Ho, C.T.; Zhang, J.; Wan, X.; Zhang, K.; Lim, J. Antioxidants: Differing meanings in food science and health science. J. Agric. Food Chem. 2018, 66, 3063–3068.
- Bagchi, D.; Moriyama, H.; Swaroop, A. Green Coffee Bean Extract in Human Health, 1st ed.; CRC-Press: Boca Raton, CA, USA, 2016; pp. 1–254.
- Corso, M.P.; Vignoli, J.A.; Benassi, M.D.T. Development of an instant coffee enriched with chlorogenic acids. J. Food Sci. Technol. 2016, 53, 1380–1388.
- Vignoli, J.A.; Bassoli, D.G.; Benassi, M.T. Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: The influence of processing conditions and raw material. Food Chem. 2011, 124, 863–868.
- Yu, L.; Nanguet, A.L.; Beta, T. Comparison of antioxidant properties of refined and whole wheat flour and bread. Antioxidants 2013, 2, 370–383.
- Prückler, M.; Siebenhandl-Ehn, S.; Apprich, S.; Höltinger, S.; Haas, C.; Schmid, E.; Kneifel, W. Wheat bran-based biorefinery 1: Composition of wheat bran and strategies of functionalization. LWT—Food Sci. Technol. 2014, 56, 211–221.
- Mukkundur Vasudevaiah, A.; Chaturvedi, A.; Kulathooran, R.; Dasappa, I. Effect of green coffee extract on rheological, physico-sensory and antioxidant properties of bread. J. Food Sci. Technol. 2017, 54, 1827–1836.
- Budryn, G.; Zyzelewicz, D.; Nebesny, E.; Oracz, J.; Krysiak, W. Influence of addition of green tea and green coffee extracts on the properties of fine yeast pastry fried products. Food Res. Int. 2013, 50, 149–160.
- Zain, M.Z.M.; Baba, A.S.; Shori, A.B. Effect of polyphenols enriched from green coffee bean on antioxidant activity and sensory evaluation of bread. J. King Saud Univ. Sci. 2018, 30, 278–282.
- Dziki, D.; Gawlik-Dziki, U.; Pecio, Ł.; Rózyło, R.; Świeca, M.; Krzykowski, A.; Rudy, S. Ground green coffee beans as a functional food supplement—Preliminary study. LWT—Food Sci. Technol. 2015, 63, 691–699.
- Hashemi Gahruie, H.; Eskandari, M.H.; Mesbahi, G.; Hanifpour, M.A. Scientific and technical aspects of yogurt fortification: A review. Food Sci. Hum. Wellness 2015, 4, 1–8.
- Rahpeyma, E.; Sekhavatizadeh, S.S. Effects of encapsulated green coffee extract and canola oil on liquid Kashk quality. Foods Raw Mater. 2020, 8, 40–51.
- Dönmez, Ö.; Mogol, B.A.; Gökmen, V. Syneresis and rheological behaviors of set yogurt containing green tea and green coffee powders. J. Dairy Sci. 2017, 100, 901–907.
- Cheynier, V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005, 81, 223S–229S.
- Sęczyk, Ł.; Świeca, M.; Gawlik-Dziki, U. Soymilk enriched with green coffee phenolics—antioxidant and nutritional properties in the light of phenolics-food matrix interactions. Food Chem. 2017, 223, 1–7.
This entry is offline, you can click here to edit this entry!
