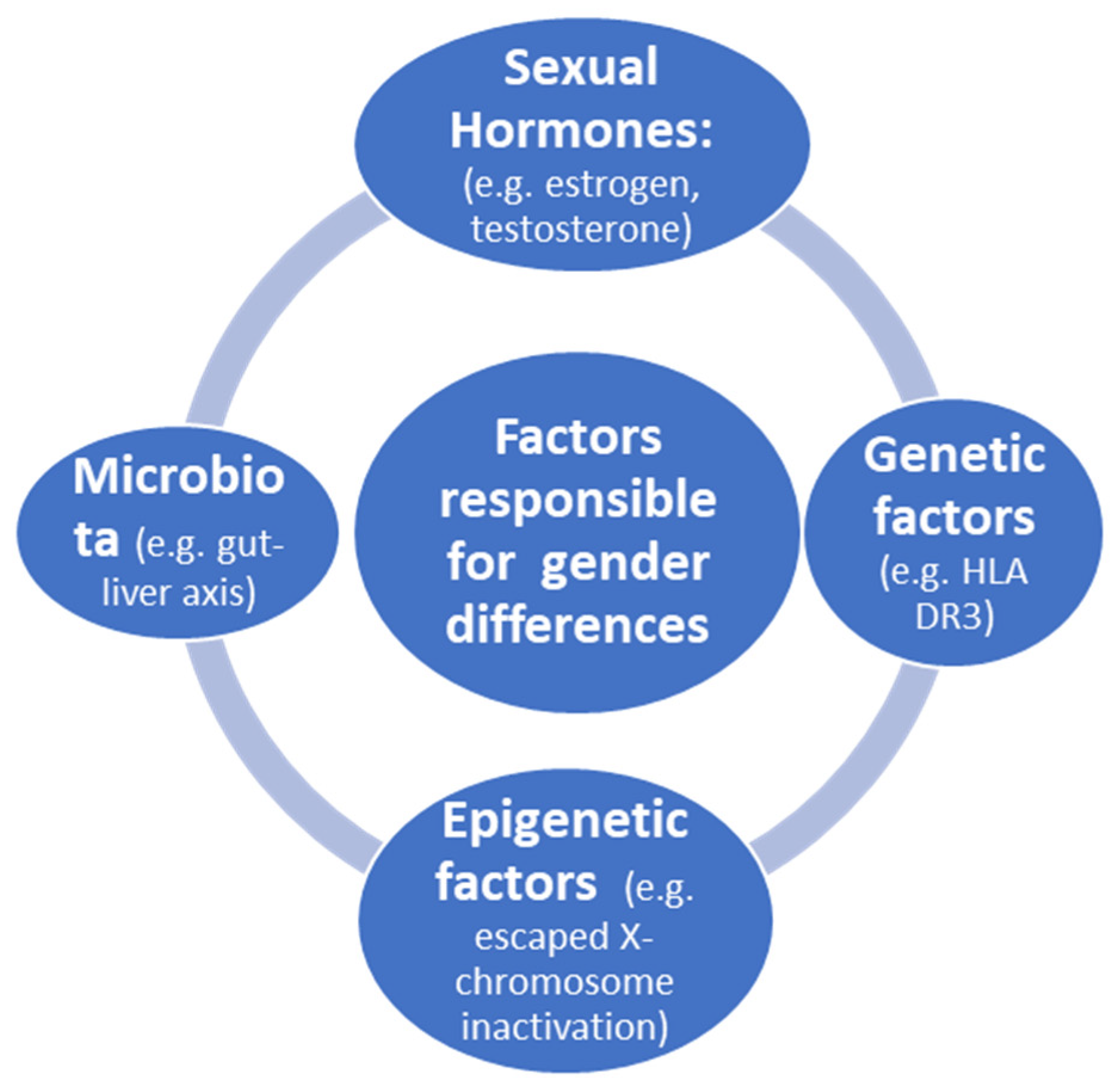
Autoimmune liver diseases (AILDs) include autoimmune hepatitis, primary biliary cholangitis and primary sclerosing cholangitis. The etiologies of AILD are not well understood but appear to involve a combination of genetic and environmental factors. AILDs commonly affect young individuals and are characterized by a highly variable clinical course. These diseases significantly influence quality of life and can progress toward liver decompensation or the onset of hepatocellular or cholangiocarcinoma; a significant number of patients eventually progress to end-stage liver disease, requiring liver transplantation.
- gender
- autoimmune liver diseases
- autoimmune hepatitis primary biliary cirrhosis
- primary sclerosing cholangitis
- overlap syndromes
- liver transplant
1. Autoimmune Hepatitis
2. Primary Biliary Cholangitis
Primary Sclerosing Cholangitis
3. Overlap Syndromes and Gender
4. Conclusions
This entry is adapted from the peer-reviewed paper 10.3390/jpm12060925
References
- Coss Adame, E.; Granados, J.; Uribe, M.; Torre, A. Does HLA-DR7 differentiate the overlap syndrome of auto-immune hepatitis-primary biliary cirrhosis (AIH-PBC) from those with auto-immune hepatitis type 1? Ann. Hepatol. 2011, 10, 28–32.
- Manns, M.P.; Czaja, A.J.; Gorham, J.D.; Krawitt, E.L.; Mieli-Vergani, G.; Vergani, D.; Vierling, J.M. Diagnosis and management of autoimmune hepatitis. Hepatology 2010, 51, 2193–2219.
- Gregorio, G.V.; Portmann, B.; Reid, F.; Donaldson, P.T.; Doherty, D.G.; McCartney, M.; Mowat, A.P.; Vergani, D.; Milei-Vergani, G. Autoimmune hepatitis in childhood: A 20-year experience. Hepatology 1997, 25, 541–547.
- Radhakrishnan, K.R.; Alkhouri, N.; Worley, S.; Arrigain, S.; Hupertz, V.; Kay, M.; Yerian, L.; Wyllie, R.; Feldstein, A.E. Autoimmune hepatitis in children—Impact of cirrhosis at presentation on natural history and long-term outcome. Dig. Liver Dis. 2010, 42, 724–728.
- Deneau, M.; Jensen, M.K.; Holmen, J.; Williams, M.S.; Book, L.S.; Guthery, S.L. Primary sclerosing cholangitis, autoimmune hepatitis, and overlap in Utah children: Epidemiology and natural history. Hepatology 2013, 58, 1392–1400.
- Jimenez-Rivera, C.; Ling, S.C.; Ahmed, N.; Yap, S.; Aglipay, M.; Borrowman, N.; Graitson, S.; Critch, J.; Rashid, M.; Ng, V.L.; et al. Incidence and characteristics of autoimmune hepatitis. Pediatrics 2015, 136, e1237–e1248.
- Czaja, A.J.; Carpenter, H.A.; Santrach, P.J.; Moore, S.B.; Taswell, H.F.; Homburger, H.A. Evidence against hepatitis viruses as important causes of severe autoimmune hepatitis in the United States. J. Hepatol. 1993, 18, 342–352.
- Boberg, K.M.; Aadland, E.; Jahnsen, J.; Raknerud, N.; Stiris, M.; Bell, H. Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand. J. Gastroenterol. 1998, 33, 99–103.
- Werner, M.; Prytz, H.; Ohlsson, B.; Almer, S.; Bjornsson, E.; Berquist, A.; Wallerstedt, S.; Sandberg-Gertzén, H.; Hultcrantz, R.; Sangfelt, P.; et al. Epidemiology and the initial presentation of autoimmune hepatitis in Sweden: A nationwide study. Scand. J. Gastroenterol. 2008, 43, 1232–1240.
- Ngu, J.H.; Bechly, K.; Chapman, B.A.; Burt, M.J.; Barclay, M.L.; Gearry, R.B.; Stedman, C.A.M. Population-based epidemiology study of autoimmune hepatitis: A disease of older women? J. Gastroenterol. Hepatol. 2010, 25, 1681–1686.
- Gronbaek, L.; Vilstrup, H.; Jepsen, P. Autoimmune hepatitis in Denmark: Incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J. Hepatol. 2014, 60, 612–617.
- Van Gerven, N.M.; Vermer, N.J.; Witte, B.I.; van Erpecum, K.J.; van Buuren, H.R.; Maijers, I.; Visscher, A.P.; Verschuren, E.C.; van Hoek, B.; Coenraad, M.J.; et al. Epidemiology and clinical characteristics of autoimmune hepatitis in the Netherlands. Scand. J. Gastroenterol. 2014, 49, 1245–1254.
- Tanaka, A.; Mori, M.; Matsumoto, K.; Ohiza, H.; Takazuma, S.; Takikawa, H. Increase trend in the prevalence and to male-to-female ratio of primary biliary cholangitis, autoimmune hepatitis and primary sclerosing cholangitis in Japan. Hepatol. Res. 2019, 49, 881–889.
- Muratori, P.; Carbone, M.; Stangos, G.; Perini, L.; Lalanne, C.; Ronca, V.; Cazzagon, N.; Bianchi, G.; Lenzi, M.; Floreani, A.; et al. Clinical and prognostic implications of acute onset of autoimmune hepatitis: An Italian multicentre study. Dig. Liver Dis. 2018, 50, 698–702.
- Straub, R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007, 28, 521–574.
- Liu, H.Y.; Buenafe, A.C.; Matejuk, A.; Ito, A.; Zamora, A.; Dwyer, J.; Vandenbark, A.A.; Offner, H. Estrogens inhibition of EAE involves effects on dendritic cell function. J. Neurosci. Res. 2002, 70, 238–248.
- Asselta, R.; Paraboschi, E.M.; Gerussi, A.; Cordell, H.J.; Mells, G.F.; Sandford, R.N.; Jones, D.E.; Nakamura, M.; Ueno, K.; Hitomi, Y. X chromosome contribution to the genetic architecture of primary biliary cholangitis. Gastroenterology 2021, 160, 2483–2495.
- Liu, Q.; Li, Y.; Ma, X.; Tang, R. Epigenetics of autoimmune liver diseases: Current progress and future directions. J. Bio-X Res. 2019, 2, 46–55.
- Pinheiro, I.; Dejager, L.; Libert, C. X-chromosome-located microRNAs in immunity: Might they explain male/female differences? Bioassays 2011, 33, 791–802.
- Di Palo, A.; Siniscalchi, C.; Salerno, M.; Russo, A.; Gravholt, C.H.; Potenza, N. What microRNAs could tell us about the X chromosome. Cell Mol. Life Sci. 2020, 77, 4069–4080.
- Chen, L.; Lu, F.-B.; Chen, D.-Z.; Wu, J.-L.; Hu, E.; Xu, L.M.; Zheng, M.-H.; Li, H.; Huang, Y.; Jin, X.-Y. BMSCs-derived miR-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis. Mol. Immunol. 2018, 93, 38–46.
- Ye, D.; Zhang, T.; Lou, G.; Liu, Y. Role of miR-223 in the pathophysiology of liver diseases. Exp. Mol. Med. 2018, 50, 128.
- Huang, C.; Xing, X.; Xiang, X.; Fan, X.; Men, R.; Ye, T.; Yang, L. MicroRNAs in autoimmune liver diseases: From diagnosis to potential therapeutic targets. Biomed. Pharmacother. 2020, 13, 110558.
- De Luca, F.; Shoenfeld, Y. The microbiome in autoimmune diseases. Clin. Exp. Immunol. 2019, 195, 74–85.
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506.
- Tansel, A.; Katz, L.H.; El-Serag, H.B.; Thrift, A.P.; Parepally, M.; Shakhatreh, M.H.; Kanwal, F. Incidence and determinants of hepatocellular carcinoma in autoimmune hepatitis: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2017, 15, 1207–1217.
- Czaja, A.J. Hepatocellular carcinoma and other malignancies in autoimmune hepatitis. Dig. Dis. Sci. 2013, 58, 1459–1476.
- Yeoman, A.D.; Al-Chalabi, T.; Karani, J.B.; Quaglia, A.; Devlin, J.; Mieli-Vergani, G.; Bomford, A.; O’Grady, J.G.; Harrison, P.M.; Heneghan, M.A. Evaluation of risk factors in the development of hepatocellular carcinoma in autoimmune hepatitis: Implication for follow-up and screening. Hepatology 2008, 48, 863–870.
- Al-Chalabi, T.; Underhill, J.A.; Portmann, B.C.; McFarlane, I.G.; Heneghan, M.A. Impact of gender on the long-term survival of patients with autoimmune hepatitis. J. Hepatol. 2008, 48, 140–147.
- Kirstein, M.M.; Metzler, F.; Geiger, E.; Einrich, E.; Hallensleben, M.; Manns, M.P.; Vogel, A. Prediction of short- and long-term outcome in patients with autoimmune hepatitis. Hepatology 2015, 62, 1524–1535.
- Carbone, M.; Mells, G.F.; Pells, G.; Dawwas, M.F.; Newton, J.L.; Heneghan, M.A.; Neuberger, J.M.; Day, D.B.; Ducker, S.J.; UK PBC Consortium; et al. Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid. Gastroenterology 2013, 144, 560–569.e7.
- Ali, A.H.; Sinakos, E.; Silveira, M.G.; Jorgensen, R.A.; Angulo, P.; Lindor, K.D. Varices in early histological stage primary biliary cirrhosis. J. Clin. Gastroenterol. 2011, 45, e66–e71.
- Marschall, H.U.; Henriksson, I.; Lindberg, S.; Söderdahl, F.; Thuresson, M.; Wahlin, S.; Ludvigsson, J.F. Incidence, prevalence, and outcome of primary biliary cholangitis in a nationwide Swedish population-based cohort. Sci. Rep. 2019, 9, 11525.
- Drazilova, S.; Babinska, I.; Gazda, J.; Halanova, M.; Janicko, M.; Kucinsky, B.; Safcak, D.; Martinkova, D.; Tarbajova, L.; Cekanova, A.; et al. Epidemiology and clinical course of primary biliary cholangitis in Eastern Slovakia. Int. J. Public Health 2020, 65, 683–691.
- Chen, S.; Duan, W.; Li, M.; Li, S.; Lv, T.; Tian, Q.; Wang, Q.; Wu, X.; Zhao, X.; Wang, X.; et al. Prognosis of 732 ursodeoxycholic acid-treated patients with primary biliary cholangitis: A single center follow-up study from China. J. Gastroenterol. Hepatol. 2019, 34, 1236–1241.
- Abdulkarim, M.; Zenouzi, R.; Sebode, M.; Schulz, L.; Quaas, A.; Lohse, A.W.; Schramm, C.; Weiler-Normann, C. Sex differences in clinical presentation and prognosis in patients with primary biliary cholangitis. Scand. J. Gastroenterol. 2019, 54, 1391–1396.
- Yagi, M.; Tanaka, A.; Abe, M.; Namisaki, T.; Yoshiji, H.; Takahashi, A.; Ohira, H.; Komori, A.; Yamagiwa, S.; Kikuchi, K.; et al. Symptoms and health-related quality of life in Japanese patients with primary biliary cholangitis. Sci. Rep. 2018, 8, 12542.
- Cheung, K.S.; Seto, W.K.; Fung, J.; Lai, C.L.; Yuen, M.F. Epidemiology and Natural History of Primary Biliary Cholangitis in the Chinese: A Territory-Based Study in Hong Kong between 2000 and 2015. Clin. Transl. Gastroenterol. 2017, 8, e116.
- Gatselis, N.K.; Zachou, K.; Lygoura, V.; Azariadis, K.; Arvaniti, P.; Spyrou, E.; Papadamou, G.; Koukoulis, G.K.; Dalekos, G.N.; Rigopoulou, E.I. Geoepidemiology, clinical manifestations and outcome of primary biliary cholangitis in Greece. Eur. J. Intern. Med. 2017, 42, 81–88.
- Kim, K.A.; Ki, M.; Choi, H.Y.; Kim, B.H.; Jang, E.S.; Jeong, S.H. Population-based epidemiology of primary biliary cirrhosis in South Korea. Aliment. Pharmacol. Ther. 2016, 43, 154–162.
- Boonstra, K.; Bokelaar, R.; Stadhouders, P.H.; Tuynman, H.A.; Poen, A.C.; van Nieuwkerk, K.M.; Witteman, E.M.; Hamann, D.; Witteman, B.J.; Beuers, U.; et al. Increased cancer risk in a large population-based cohort of patients with primary biliary cirrhosis: Follow-up for up to 36 years. Hepatol. Int. 2014, 8, 266–274.
- Younossi, Z.M.; Stepanova, M.; Golabi, P.; Epstein, R.S.; Strauss, M.E.; Nader, F.; Racila, A. Factors Associated with Potential Progressive Course of Primary Biliary Cholangitis: Data from Real-world US Database. J. Clin. Gastroenterol. 2019, 53, 693–698.
- Marzioni, M.; Bassanelli, C.; Ripellino, C.; Urbinati, D.; Alvaro, D. Epidemiology of primary biliary cholangitis in Italy: Evidence from a real-world database. Dig. Liver Dis. 2019, 51, 724–729.
- Lammers, W.J.; Hirschfield, G.M.; Corpechot, C.; Nevens, F.; Lindor, K.D.; Janssen, H.L.; Floreani, A.; Ponsioen, C.Y.; Mayo, M.J.; Invernizzi, P.; et al. Development and Validation of a Scoring System to Predict Outcomes of Patients with Primary Biliary Cirrhosis Receiving Ursodeoxycholic Acid Therapy. Gastroenterology 2015, 149, 1804–1812.e4.
- Zhang, L.N.; Shi, T.Y.; Shi, X.H.; Wang, L.; Yang, Y.J.; Liu, B.; Gao, L.X.; Shuai, Z.W.; Kong, F.; Chen, H.; et al. Early biochemical response to ursodeoxycholic acid and long-term prognosis of primary biliary cirrhosis: Results of a 14-year cohort study. Hepatology 2013, 58, 264–272.
- John, B.V.; Aitcheson, G.; Schwartz, K.B.; Khakoo, N.S.; Dahman, B.; Deng, Y.; Goldberg, D.; Martin, P.; Taddei, T.H.; Levy, C.; et al. Male Sex Is Associated with Higher Rates of Liver-Related Mortality in Primary Biliary Cholangitis and Cirrhosis. Hepatology 2021, 74, 879–891.
- Cheung, A.C.; Lammers, W.J.; Murillo Perez, C.F.; van Buuren, H.R.; Gulamhusein, A.; Trivedi, P.J.; Lazaridis, K.N.; Ponsioen, C.Y.; Floreani, A.; Hirschfield, G.M.; et al. Effects of Age and Sex of Response to Ursodeoxycholic Acid and Transplant-free Survival in Patients with Primary Biliary Cholangitis. Clin. Gastroenterol. Hepatol. 2019, 17, 2076–2084.e2.
- Lammert, C.; Juran, B.D.; Schlicht, E.; Chan, L.L.; Atkinson, E.J.; de Andrade, M.; Lazaridis, K.N. Biochemical response to ursodeoxycholic acid predicts survival in a North American cohort of primary biliary cirrhosis patients. J. Gastroenterol. 2014, 49, 1414–1420.
- Tian, S.; Liu, Y.; Sun, K.; Zhou, X.; Ma, S.; Zhang, M.; Zhou, X.; Wang, L.; Han, Y. A nomogram based on pretreatment clinical parameters for the prediction of inadequate biochemical response in primary biliary cholangitis. J. Clin. Lab. Anal. 2020, 34, e23501.
- Melchor-Mendoza, Y.K.; Martínez-Benítez, B.; Mina-Hawat, A.; Rodríguez-Leal, G.; Duque, X.; Moran-Villota, S. Ursodeoxycholic Acid Therapy in Patients with Primary Biliary Cholangitis with Limited Liver Transplantation Availability. Ann. Hepatol. 2017, 16, 430–435.
- Delgado, J.S.; Vodonos, A.; Delgado, B.; Jotkowitz, A.; Rosenthal, A.; Fich, A.; Novack, V. Primary biliary cirrhosis in Southern Israel: A 20 year follow up study. Eur. J. Intern. Med. 2012, 23, e193–e198, Erratum in Lancet 2015, 386, 1536.
- Chen, J.; Xue, D.; Gao, F.; Tao, L.; Li, Y.; Zhang, Q.; Wang, R.; Sun, L.; Yang, X.; Liu, Y.; et al. Influence factors and a predictive scoring model for measuring the biochemical response of primary biliary cholangitis to ursodeoxycholic acid treatment. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1352–1360.
- Madir, A.; Božin, T.; Mikolašević, I.; Milić, S.; Štimac, D.; Mijić, M.; Filipec Kanižaj, T.; Biloglav, Z.; Lucijanić, M.; Lucijanić, I.; et al. Epidemiological and clinical features of primary biliary cholangitis in two Croatian regions: A retrospective study. Croat. Med. J. 2019, 60, 494–502.
- Cortez-Pinto, H.; Liberal, R.; Lopes, S.; Machado, M.V.; Carvalho, J.; Dias, T.; Santos, A.; Agostinho, C.; Figueiredo, P.; Loureiro, R.; et al. Predictors for incomplete response to ursodeoxycholic acid in primary biliary cholangitis. Data from a national registry of liver disease. United Eur. Gastroenterol. J. 2021, 9, 699–706.
- European Association for the Study of the Liver. Clinical Practice Guidelines: The Diagnosis and Management of Patients with Primary Biliary Cholangitis. J. Hepatol. 2017, 67, 145–172.
- Roberts, S.B.; Ismail, M.; Kanagalingam, G.; Mason, A.L.; Swain, M.G.; Vincent, C.; Yoshida, E.M.; Tsien, C.; Flemming, J.A.; Janssen, H.L.A.; et al. Real-World Effectiveness of Obeticholic Acid in Patients with Primary Biliary Cholangitis. Hepatol. Commun. 2020, 4, 1332–1345.
- Gomez, E.; Garcia Buey, L.; Molina, E.; Casado, M.; Conde, I.; Berenguer, M.; Jorquera, F.; Simón, M.A.; Olveira, A.; Hernández-Guerra, M.; et al. Effectiveness and safety of obeticholic acid in a Southern European multicentre cohort of patients with primary biliary cholangitis and suboptimal response to ursodeoxycholic acid. Aliment Pharmacol. Ther. 2021, 53, 519–530.
- Harms, M.H.; Hirschfield, G.M.; Floreani, A.; Mayo, M.J.; Parés, A.; Liberman, A.; Malecha, E.S.; Pencek, R.; MacConell, L.; Hansen, B.E. Obeticholic acid is associated with improvements in AST-to-platelet ratio index and GLOBE score in patients with primary biliary cholangitis. JHEP Rep. 2020, 3, 100191.
- D’Amato, D.; De Vincentis, A.; Malinverno, F.; Viganò, M.; Alvaro, D.; Pompili, M.; Picciotto, A.; Palitti, V.P.; Russello, M.; Storato, S.; et al. Real-world experience with obeticholic acid in patients with primary biliary cholangitis. JHEP Rep. 2021, 3, 100248.
- Natarajan, Y.; Tansel, A.; Patel, P.; Emologu, K.; Shukla, R.; Qureshi, Z.; El-Serag, H.B.; Thrift, A.P.; Kanwal, F. Incidence of Hepatocellular Carcinoma in Primary Biliary Cholangitis: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2021, 66, 2439–2451.
- Trivedi, P.J.; Lammers, W.J.; van Buuren, H.R.; Parés, A.; Floreani, A.; Janssen, H.L.; Invernizzi, P.; Battezzati, P.M.; Ponsioen, C.Y.; Corpechot, C.; et al. Stratification of hepatocellular carcinoma risk in primary biliary cirrhosis: A multicentre international study. Gut 2016, 65, 321–329.
- Harada, K.; Hirohara, J.; Ueno, Y.; Nakano, T.; Kakuda, Y.; Tsubouchi, H.; Ichida, T.; Nakanuma, Y. Incidence of and risk factors for hepatocellular carcinoma in primary biliary cirrhosis: National data from Japan. Hepatology 2013, 57, 1942–1949.
- Adejumo, A.C.; Akhtar, D.H.; Dennis, B.B.; Cholankeril, G.; Alayo, Q.; Ogundipe, O.A.; Kim, D.; Ahmed, A. Gender and Racial Differences in Hospitalizations for Primary Biliary Cholangitis in the USA. Dig. Dis. Sci. 2021, 66, 1461–1476.
- Fan, X.; Wang, T.; Shen, Y.; Xi, X.; Yang, L. Underestimated Male Prevalence of Primary Biliary Cholangitis in China: Results of a 16-yr cohort study involving 769 patients. Sci. Rep. 2017, 7, 6560.
- Lleo, A.; Jepsen, P.; Morenghi, E.; Carbone, M.; Moroni, L.; Battezzati, P.M.; Podda, M.; Mackay, I.R.; Gershwin, M.E.; Invernizzi, P. Evolving Trends in Female to Male Incidence and Male Mortality of Primary Biliary Cholangitis. Sci. Rep. 2016, 6, 25906.
- Lu, M.; Zhou, Y.; Haller, I.V.; Romanelli, R.J.; VanWormer, J.J.; Rodriguez, C.V.; Anderson, H.; Boscarino, J.A.; Schmidt, M.A.; Daida, Y.G.; et al. Increasing Prevalence of Primary Biliary Cholangitis and Reduced Mortality with Treatment. Clin. Gastroenterol. Hepatol. 2018, 16, 1342–1350.e1.
- Sayiner, M.; Golabi, P.; Stepanova, M.; Younossi, I.; Nader, F.; Racila, A.; Younossi, Z.M. Primary Biliary Cholangitis in Medicare Population: The Impact on Mortality and Resource Use. Hepatology 2019, 69, 237–244.
- Lindkvist, B.; Benito de Valle, M.; Gullberg, B.; Bjornsson, E. Incidence and Prevalence of Primary SclerosingCholangitis in a Defined Adult Population in Sweden. Hepatology 2010, 52, 571–577.
- Lamba, M.; Hieng Ngu, J.; Stedman, C.A.M. Trends in Incidence of Autoimmune Liver Diseases and Increasing Incidence of Autoimmune Hepatitis. Clin. Gastroenterol. Hepatol. 2020, 19, 573–579.e1.
- Bakhshi, Z.; Hilscher, M.B.; Gores, G.J.; Harmsen, W.S.; Viehman, J.K.; LaRusso, N.F.; Gossard, A.A.; Lazaridis, K.N.; Lindor, K.D.; Eaton, J.E. An update on primary sclerosing cholangitis epidemiology, outcomes and quantification of alkaline phosphatase variability in a population-based cohort. J. Gastroenterol. 2020, 55, 523–532.
- Carbone, M.; Kodra, Y.; Rocchetti, A.; Manno, V.; Minelli, G.; Gerussi, A.; Ronca, V.; Malinverno, F.; Cristoferi, L.; Floreani, A.; et al. Primary Sclerosing Cholangitis: Burden of Disease and Mortality Using Data from the National Rare Diseases Registry in Italy. Int. J. Environ. Res. Public Health 2020, 17, 3095.
- Trivedi, P.J.; Crothers, H.; Mytton, J.; Bosch, S.; Iqbal, T.; Ferguson, J.; Hirschfield, G.M. Effects of Primary Sclerosing Cholangitis on Risks of Cancer and Death in People with Inflammatory Bowel Disease, Based on Sex, Race, and Age. Gastroenterology 2020, 159, 915–928.
- Rizvi, S.; Gores, G.J. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013, 145, 1215–1229.
- Jaiswal, M.; La Russo, N.F.; Burgart, L.J.; Gores, G.J. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000, 60, 184–190.
- Boonstra, K.; Weersma, R.K.; van Erpecum, K.J.; Rauws, E.A.; Spanier, B.W.; Poen, A.C.; van Nieuwkerk, K.M.; Drenth, J.P.; Witteman, B.J.; Tuynman, H.A.; et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 2013, 58, 2045–2055.
- Kornfeld, D.; Ekbom, A.; Ihre, T. Survival and risk of cholangiocarcinoma in patients with primary sclerosing cholangitis. A population-based study. Scand. J. Gastroenterol. 1997, 32, 1042–1045.
- Weismüller, T.J.; Trivedi, P.J.; Bergquist, A.; Imam, M.; Lenzen, H.; Ponsioen, C.Y.; Holm, K.; Gotthardt, D.; Färkkilä, M.A.; Marschall, H.U.; et al. Patient Age, Sex, and Inflammatory Bowel Disease Phenotype Associate with Course of Primary Sclerosing Cholangitis. Gastroenterology 2017, 152, 1975–1984.e8.
- Soetikno, R.M.; Lin, O.S.; Heidenreich, P.A.; Young, H.S.; Blackstone, M.O. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: A meta-analysis. Gastrointest. Endosc. 2002, 56, 48–54.
- Zheng, H.H.; Jiang, X.L. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: A meta-analysis of 16 observational studies. Eur. J. Gastroenterol. Hepatol. 2016, 28, 383–390.
- Karlsen, T.H. Primary sclerosing cholangitis: 50 years of a gut-liver relationship and still no love? Gut 2016, 65, 1579–1581.
- Rupp, C.; Rössler, A.; Zhou, T.; Rauber, C.; Friedrich, K.; Wannhoff, A.; Weiss, K.H.; Sauer, P.; Schirmacher, P.; Süsal, C.; et al. Impact of age at diagnosis on disease progression in patients with primary sclerosing cholangitis. United Eur. Gastroenterol. J. 2018, 6, 255–262.
- Guerra, I.; Bujanda, L.; Castro, J.; Merino, O.; Tosca, J.; Camps, B.; Gutiérrez, A.; Gordillo Ábalos, J.; de Castro, L.; Iborra, M.; et al. Clinical Characteristics, Associated Malignancies and Management of Primary Sclerosing Cholangitis in Inflammatory Bowel Disease Patients: A Multicentre Retrospective Cohort Study. J. Crohns Colitis 2019, 13, 1492–1500.
- Carey, E.J.; Ali, A.H.; Lindor, K.D. Primary biliary cirrhosis. Lancet 2015, 386, 1565–1575.
- Floreani, A.; Franceschet, I.; Cazzagon, N. Primary biliary cirrhosis: Overlaps with other autoimmune disorders. Semin. Liver Dis. 2014, 34, 352–360.
- Freedman, B.L.; Danford, C.J.; Patwardhan, V.; Bonder, A. Treatment of overlap syndromes in autoimmune liver disease: A systematic review and meta-analysis. J. Clin. Med. 2020, 9, 1449.
- Gregorio, G.V.; Portmann, B.; Karani, J.; Harrison, P.; Donaldson, P.T.; Vergani, D.; Mieli-Vergani, G. Autoimmune hepatitis/sclerosing cholangitis overlap syndrome in childhood: A 16-year prospective study. Hepatology 2001, 33, 544–553.
- Abdalian, R.; Dhar, P.; Jhaveri, K.; Haider, M.; Guindi, M.; Heathcote, E.J. Prevalence of sclerosing cholangitis in adults with autoimmune hepatitis: Evaluating the role of routine magnetic resonance imaging. Hepatology 2008, 47, 949–957.
- Al-Chalabi, T.; Portmann, B.C.; Bernal, W.; McFarlane, I.G.; Heneghan, M.A. Autoimmune hepatitis overlap syndromes: An evaluation of treatment response, long-term outcome and survival. Aliment Pharmacol. Ther. 2008, 28, 209–220.
- Gheorghe, L.; Iacob, S.; Gheorghe, C.; Iacob, R.; Simionov, I.; Vadan, R.; Becheanu, G.; Parvulescu, I.; Toader, C. Frequency and predictive factors for overlap syndrome between autoimmune hepatitis and primary cholestatic liver disease. Eur. J. Gastroenterol. Hepatol. 2004, 16, 585–592.
- Lüth, S.; Kanzler, S.; Frenzel, C.; Kasper, H.U.; Dienes, H.P.; Schramm, C.; Galle, P.R.; Herkel, J.; Lohse, A.W. Characteristics and long-term prognosis of the autoimmune hepatitis/primary sclerosing cholangitis overlap syndrome. J. Clin. Gastroenterol. 2009, 43, 75–80.
- Chayanupatkul, M.; Fiel, M.I.; Schiano, T.D. The clinical characteristics, pre- and post-liver transplantation outcomes in patients having autoimmune overlap syndromes. Clin. Transplant. 2020, 34, e13841.
