Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Dentistry, Oral Surgery & Medicine
The increased use of dental implants in oral rehabilitation has been followed by the development of new biomaterials as well as improvements in the performance of biomaterials already in use inspired by the properties of the tissues to be replaced. This triggers the need for appropriate analytical approaches to assess the biological and, ultimately, clinical benefits of these approaches.
- implant surface
- osseointegration
- dentistry
- oral surgery
- oral rehabilitation
- biomimetic
- dental implants
1. Introduction
In recent years, dental implants have been widely used to replace missing teeth in order to restore the natural functions of the stomatognathic system through appropriate mechanical properties, stability, adequate bone integration, and regeneration [1][2][3].
These medical interventions have progressively changed to meet patient needs and improve their quality of life. However, research has focused on finding simpler, faster surgical procedures that allow for multiple restorative options with improved aesthetics and precise prosthetic components that offer procedural safety [4][5][6].
The first documented dental implant dates back to 600 AD. Since then, various materials have been tested as dental implants, including gold, silver, porcelain, and other materials [7]. Professor Per-Ingvar Brånemark’s study in 1950 demonstrated that titanium structures could be permanently incorporated into the bone such that this interface could only be separated by fracture [8]. In this context, the term osseointegration was introduced to describe a direct structural and functional connection between living bone and the surface of an implant without the intervention of soft tissue [8][9].
Bone is a complex connective tissue composed of a mineralized matrix (30%), which provides mechanical strength, and an organic matrix (70%), providing elasticity and flexibility [10]. It is a dynamic structure that undergoes a modelling and remodelling process and plays an important role in microfracture healing and skeletal adaptation. Bone tissue consists of four cell types, two of which, osteoblasts and osteoclasts, are involved in bone resorption and bone apposition dynamics triggered by molecular stimuli, which in turn leads to the differentiation of mesenchymal stem cells (MSCs) [10][11].
The extracellular matrix (ECM) forms a structural architecture that surrounds cells and consists of four main components, fibers, multi-adhesive proteins, proteoglycan and non-proteoglycan polysaccharides, which influence the behavior of the resident cells. The cellular interactions with the extracellular matrix are a complex network that is critical to maintaining tissue homeostasis. Besides maintaining self-renewal, the ECM also regulates the differentiation ability of undifferentiated cells by modelling the activity of growth factors [11][12].
Research has focused on the long-term survival rates of dental implants [13][14], with osseointegration being the main criteria for clinical success [15]. Originally described by Professor Brånemark, the phenomenon of osseointegration is a consequence of a series of cellular and molecular events that begins immediately after implant placement due to the interactions between the implant, biological fluids and peri-implant tissues [16][17][18][19]. The osteointegration process can be divided into three phases:
- (1)
-
The initial tissue response;
- (2)
-
Peri-implant osteogenesis;
- (3)
-
Peri-implant bone remodelling.
(1) Initial tissue response: The initial process begins with the preparation of the drill hole and implant placement. From 0 to 4 h after surgical trauma, calcium ions and plasma proteins adhere to the implant surface [11]. The surgical trauma leads to an inflammatory process through activation of the complement system and neutrophil infiltration, resulting in growth factor and cytokine release. Depending on the transmitted stimuli, macrophages differentiate into two different phenotypes: M1—pro-inflammatory or M2—anti-inflammatory. Clot formation, platelets, and the fibrin matrix serve as the scaffold for the migration, proliferation, and differentiation of leukocytes and mesenchymal cells. This process takes place 1 to 3 days after implant placement [20][21][22].
(2) Peri-implant osteogenesis: After 3 to 4 days of surgery, the angiogenesis activity and the reorganization of a blood clot in the granulation tissue of the cancellous bone are more evident, while a blood clot with a high number of erythrocytes and fibroblast-like cells is still present in the area adjacent to the implant. From 7 to 14 days, the MSCs differentiate into osteoblasts and produce a fibrillar noncollagenous extracellular matrix rich in calcium, phosphorus, osteopontin and bone sialoprotein, designated primary bone tissue [11][23].
(3) Peri-implant bone remodelling: The final stage of healing takes place at 2 weeks. It is characterized by an increase in primary bone apposition in direct contact with the pristine bone and implant surface [11]. The first signs of bone remodelling also appear in the primary osteoid. Osteoclasts direct the remodelling process from immature bone to highly mineralized lamellar bone by binding to the mineralized collagen matrix and creating a zone where the bone is deposited directly on the implant surface. While after 3 months of implant placement, there may be lamellar and non-lamellar bone around the implant, the process of osseointegration may take 1 year or more to complete [17][24][25].
Currently, an implant is considered osseointegrated when there is no relative movement between the implant and the bone and no symptoms under a loading force [16][17][26]. This biological fixation process is a prerequisite for any implant-supported prosthesis and its long-term success [16][19]. Although osseointegration has been extensively demonstrated from a histological and clinical perspective, our understanding of this biological process is still limited. However, some parameters that can influence the osseointegration process have already been described in the literature. These factors are related to the implant (design, shape, length, diameter, and others), the host (implant sit, bone cellularity and density, systemic diseases, and others), the surgery (tissue damage, soft tissue healing ability, speed perforation and others), healing time and exerted loads [17][27].
The success of osseointegration of dental implants has been reported in many studies; however, a systematic review of the Cochrane database found insufficient evidence for the superiority of any particular type of implant feature or system [2]. Despite all the factors that can affect osseointegration, in this work, we will focus mainly on factors related to the materials and biomimetic properties of implant surfaces (Figure 1).
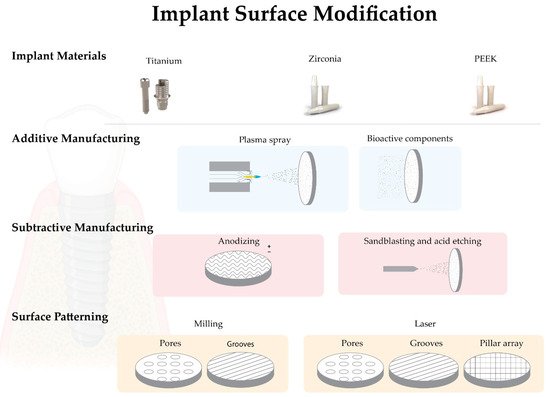
Figure 1. Schematic image of the most relevant biomimetic surface modification strategies.
2. Dental Implants Base Materials
2.1. Titanium
Pure titanium (Ti) is a transition metal with atomic number 22, a melting point of 1668 °C and a boiling point of 3287 °C. Upon contact with atmospheric air, Ti forms a thin layer of titanium oxide covering its surface; this mechanism is unique to titanium, silicon and zirconia. [28] The layer is more extensive when titanium implants are exposed to biological tissue, giving them excellent biocompatibility [17][28]. Titanium can be used as a pure metal or as an alloy containing other metals such as vanadium, aluminium, niobium, iron, magnesium or zirconium. According to the American Society for Testing and Materials (ASTM), there are 4 Ti classes, depending on the amount of oxygen, nitrogen, hydrogen and carbon introduced during the purification process. There is also Grade V, which is a titanium aluminum alloy with 4% vanadium (Ti6AL4V) [29].
Commercially, Ti is most commonly used for dental implants, particularly in the IV grade, which is the highest strength of the various grades of pure titanium, excluding alloys [30]. Titanium and its alloys are the material of choice for the manufacture of dental implants due to their excellent biocompatibility, corrosion resistance, elasticity modulus and excellent mechanical properties [8][17][31][32], which are important for long-term implant success [19]. Several studies have therefore reported high success rates of titanium implants. Disadvantages such as hypersensitivity reactions, the difference in the elasticity modulus, wear resistance, electrical conductivity and the grey colour of this material are all of concern. Alternative materials have been developed to provide biological stability with better or comparable mechanical properties [31] to improve the biological and mechanical properties of these dental implants.
2.2. Zirconia
Zirconia (ZrO2) is a crystalline oxide form of zirconium, a transition metal with atomic number 44. Structurally, zirconia is a polymorphic material that occurs in three forms: monoclinic, tetragonal, and cubic. During the cooling process, some microcracks may appear. To avoid this, oxides such as magnesium oxide (MgO), yttrium oxide (Y2O3), calcium oxide (CaO) and cerium oxide (Ce2O3) are added to keep the tetragonal structure at room temperature and control the stress [33][34].
Tetragonal polycrystalline zirconia (TZP) is a zirconia-based ceramic predominantly in the tetragonal phase and generally stabilized with yttrium oxide (3–6% by weight), referred to yttria-stabilized polycrystalline tetragonal zirconia (YTZP). The opaque and white colour of YTZP, together with high fracture strength, flexural strength, thermal stability, low thermal conductivity, chemical resistance, biocompatibility and low affinity for bacterial colonization, renders this material a good candidate for implant dentistry. Overall, different studies have found that zirconia and titanium have similar bone tissue integration [34][35]. However, despite technological advances in the manufacture of ceramics, the mechanical behavior of zirconia has limitations, namely the ageing process. This is a process involving the degradation of zirconia at low temperatures that leads to the formation of cracks in the ceramic and subsequently fracture [34].
A recent systematic and critical review suggested that zirconia implants are a promising alternative to titanium based on its superior soft tissue behavior, biocompatibility and aesthetics while maintaining the same osseointegration ability as titanium [35][36]. The literature indicates that treated zirconia surfaces exhibit better or similar clinical bone-to-implant interface outcomes compared to equivalent titanium surfaces [34][36][37]. However, other systematic reviews show contradictory results favouring the titanium surface’s behavior [2][38]. In these reviews, the evaluation of surface parameters was not considered as a variable influencing material behavior. Therefore, these findings need to be confirmed by integrating adequate characterization of the surfaces.
3. Biomimetic Surface Properties
3.1. Topography
The implant geometry has continuously changed and evolved over the years. Numerous reports have shown that the macro geometry of implants can affect the osseointegration process, such as good primary stability, implant sealing, and maintenance of marginal bone level [39][40][41].
The surface topography can be divided into three levels according to the scale: macro, micro and nano. Macrotopography is defined in a scale range from 10 μm to mm, and is found in most implants commercially available today with a cylindrical shape and thread design, which may play a key role in increasing implant stability [19][27]. In terms of microtopography, the scale range is 1–10 μm, which appears to accelerate and increase bone-to-implant contact, maximize adhesion between the mineralized bone and the implant surface, and provide more predictable long-term clinical outcomes [42][43]. While the scale range defined for nanotopography is between 1 and 100 nm, it is believed to play an important role in protein adsorption and cell adhesion. Most of these studies are performed in preclinical models that lack clinical validation since their exact function in vivo is unknown [44][45].
It is now known that surface topography is one of the key biomimetic factors that can directly affect the proliferation, structure, and alignment of human cells and their function and is also considered to be a critical determinant of cell adhesion [46][47][48]. However, there is no consensus on which physical topography or characteristic dimensions might be relevant for biomedical applications [39][49][50].
3.2. Roughness
Rough implants affect the response of osteogenic and inflammatory cells by increasing bone-to-implant contact and overall clinical success, with faster healing rates and potential for earlier loading times [4][11][51]. Several researchers have expressed interest in the directionally rough implant surface, particularly in animal studies that have shown superior osseointegration of rough surfaces compared to smooth or machined surfaces [45]. However, depending on the method used, roughened surfaces with different topographical properties can be generated, which can be an issue in terms of the definition of rough or smooth surfaces.
The three most commonly used methods to measure implant surface roughness are: contact profilometry, optical profilometry, and contact atomic microscopy [52][53][54]. Various parameters such as Ra and Rz can be used to assess surface roughness. Ra is an arithmetic mean between the highest and lowest points on the surface, and Rz is calculated by measuring the vertical distance between the highest and lowest points on the surface [45].
4. Implant Surface Modifications
4.1. Biomimetic Surface Modifications—Additive Manufacturing
4.1.1. Plasma Spray
Plasma spray is a typical additive modification used on titanium surfaces (titanium plasma spray—TPS) that increases the surface roughness through hydroxyapatite deposition [29][55]. In this technique, the particles are injected into a plasma torch at high temperatures, projected onto the implant surface, and allowed to condense and merge. To ensure excellent durability of the coating, the surface is usually sandblasted, and the final coating obtained can range in thickness from a few micrometers to millimeters. It can also be used to obtain surface roughness with Sa > 2 μm [19][29]. Some clinical complications associated with surfaces, such as delamination and marginal bone resorption, have been reported [55][56]. Today there is a consensus on the clinical benefits of using moderately rough implants instead of plasma-sprayed surfaces [19].
4.1.2. Addition of Bioactive Components
The chemical properties of biomaterial surfaces play an essential role in cell-biomaterial interaction and consequently in the osseointegration process. There is a growing concern about bacterial colonization and biofilm formation on dental implants, leading to the development of new implant materials and antibacterial implant surfaces [57][58]. The addition of bioactive components to implant surfaces can be classified into two groups: one that favors cell adhesion and the osseointegration process and the other that decreases bacterial adhesion and biofilm formation [24][59][60][61].
The addition of fluoride, silver, zinc, copper and nickel particles has been suggested by several authors based on their antibacterial properties. Fluoride nanoparticles appear to have the ability to reduce bacterial colonization on the YTZP implant surface [62]. At the same time, silver, zinc, copper, and nickel have been incorporated into titanium surfaces at the level of nanotubules generated by anodization to obtain a surface with antimicrobial activity [63]. Several synthetic and natural bioactive agents have been added to the biomaterial surfaces to enhance bone healing, osseointegration, and implant integration into the peri-implant tissue [19][64]. Hydroxyapatite (HA) or beta-tricalcium phosphate (βTCP) are used as a biological layer of apatite coating that has shown good results in terms of biocompatibility, osteoblast differentiation and osseointegration. However, an in vitro study suggests that bioactive-modified titanium and zirconia surfaces reduce fibroblast cell adhesion, viability, and proliferation compared to pure biomaterial [65][66]. These layers showed low tensile strength (<51 MPa) and fracture toughness (0.28 to 1.41 MPa.m1/2). Scientists have developed a new coating method inspired by the natural biomineralization process to avoid these drawbacks. In this process, calcium phosphate crystals deposited on the titanium surface from simulated body fluids (SBF) form a coating at room temperature [67][68].
4.2. Biomimetic Surface Modifications—Subtractive Manufacturing
4.2.1. Anodizing
The titanium surface can be modified by an anodization technique using strong acids such as sulfuric acid (H2SO4), phosphoric acid (H3PO4), hydrofluoric acid (HF) or nitric acid (HNO3), which increases surface roughness and oxide layer formation [69][70]. Nowadays, one of the most popular brands uses this type of surface treatment (TiUnite, Nobel Biocare, Sweden). In animal and human studies, a higher BIC was observed for dental implants with this type of surface treatment compared to machined implants [69][71].
4.2.2. Blasting and/or Acid Etching
These subtractive procedures can be performed separately or simultaneously. Sandblasting with titanium oxide or alumina particles is another method that can increase surface roughness. Normally, the particles are thrown through an high-speed outlet nozzle [45][72]. Strong acids such as HF, HNO3, H2SO4 or HCL are used to remove oxide impurities. After acid etching, the surfaces are minimally rough with Sa values < 1 μm and a modification of the chemical composition of surfaces [19][72].
Combined sandblasting and acid etching (SBAE) is often used to modify implant surfaces. It involves sandblasting with alumina or titanium particles, followed by acid etching. The main reason for combining these methods is to create a surface with excellent roughness for mechanical fixation and with increased potential for protein adhesion [73]. When comparing implants processed with different surface treatments, SBAE implants showed a greater resistance against reverse torque. [74]. It is noteworthy that this combination, commercially supplied as SLA (Large-grit Sandblasted Acid-Etched) (Straumann, Basel, Switzerland), has shown increased osteoblastic differentiation in vitro compared to smooth surfaces [75][76][77]. However, most of the reported literature is based on surface modifications carried out on titanium, which are currently still poorly described on zirconia.
This entry is adapted from the peer-reviewed paper 10.3390/biomimetics7020074
References
- Londoño, J.J.; Ramos, A.M.; Correa, S.A.; Mesnard, M. Review of expandable dental implants. Br. J. Oral Maxillo-Facial Surg. 2021, 59, 546–554.
- Esposito, M.; Ardebili, Y.; Worthington, H.V. Interventions for replacing missing teeth: Different types of dental implants. Cochrane Database Syst. Rev. 2019, 2019, CD003815.
- Mombelli, A.; Müller, N.; Cionca, N. The epidemiology of peri-implantitis. Clin. Oral Implant. Res. 2012, 23, 67–76.
- Elias, C.N.; Oshida, Y.; Lima, J.H.C.; Muller, C.A. Relationship between surface properties (roughness, wettability and morphology) of titanium and dental implant removal torque. J. Mech. Behav. Biomed. Mater. 2008, 1, 234–242.
- Gupta, R.; Gupta, N.; Weber, K.K.; Dental Implants. StatPearls 2021. Available online: https://europepmc.org/article/nbk/nbk470448 (accessed on 13 April 2022).
- Comisso, I.; Arias-Herrera, S.; Gupta, S. Zirconium dioxide implants as an alternative to titanium: A systematic review. J. Clin. Exp. Dent. 2021, 13, e511–e519.
- Bobbio, A. The first endosseous alloplastic implant in the history of man. Bull. Hist. Dent. 1972, 20, 1–6.
- Branemark, P. Vital microscopy of bone marrow in rabbit. Scand. J. Clin. Lab. Investig. 1959, 11, 1–82.
- Brånermark, P.; Adell, R.; Albrektsson, T.; Lekholm, U.; Lundkivist, S.; Rockler, B. Osseointegrated titanium fixtures in the treatment of edentulousness. Biomaterials 1983, 4, 25–28.
- Florencio-Silva, R.; da Silva Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746.
- Pellegrini, G.; Francetti, L.; Barbaro, B.; del Fabbro, M. Novel surfaces and osseointegration in implant dentistry. J. Inves-Tigative Clin. Dent. 2018, 9, e12349.
- McMahon, M.; Ye, S.; Pedrina, J.; Dlugolenski, D.; Stambas, J. Extracellular Matrix Enzymes and Immune Cell Biology. Front. Mol. Biosci. 2021, 8, 703868.
- Esposito, M.; Grusovin, M.G.; Worthington, H. Interventions for replacing missing teeth: Treatment of peri-implantitis. Cochrane Database Syst. Rev. 2012, 1, CD004970.
- Liñares, A.; Grize, L.; Muñoz, F.; Pippenger, B.E.; Dard, M.; Domken, O.; Blanco-Carrión, J. Histological assessment of hard and soft tissues sur-rounding a novel ceramic implant: A pilot study in the minipig. J. Clin. Periodontol. 2016, 43, 538–546.
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prostho-dontics. J. Prosthodont. Res. 2016, 60, 12–19.
- Brånemark, R.; Brånemark, P.I.; Rydevik, B.; Myers, R.R. Osseointegration in skeletal reconstruction and rehabilitation: A review. J. Rehabil. Res. Dev. 2001, 38, 175–181.
- Lee, J.W.Y.; Bance, M.L. Physiology of Osseointegration. Otolaryngol. Clin. N. Am. 2019, 52, 231–242.
- Ajami, E.; Fu, C.; Wen, H.B.; Bassett, J.; Park, S.J.; Pollard, M. Early Bone Healing on Hydroxyapatite-Coated and Chemically-Modified Hydrophilic Implant Surfaces in an Ovine Model. Int. J. Mol. Sci. 2021, 22, 9361.
- le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854.
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969.
- Amengual-Peñafiel, L.; Córdova, L.A.; Jara-Sepúlveda, M.C.; Brañes-Aroca, M.; Marchesani-Carrasco, F.; Cartes-Velásquez, R. Osteoimmunology drives dental implant osseointegration: A new paradigm for implant dentistry. Jpn. Dent. Sci. Rev. 2021, 57, 12–19.
- Chawla, A. Control of Macrophage Activation and Function by PPARs. Circ. Res. 2010, 106, 1559–1569.
- Kim, J.; Adachi, T. Cell-fate decision of mesenchymal stem cells toward osteocyte differentiation is committed by spheroid culture. Sci. Rep. 2021, 11, 13204.
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57.
- Irandoust, S.; Müftü, S. The interplay between bone healing and remodeling around dental implants. Sci. Rep. 2020, 10, 4335.
- Zarb, G.A.; Schmitt, A. The longitudinal clinical effectiveness of osseointegrated dental implants in anterior partially edentulous patients. Implant Dent. 1993, 6, 189–196.
- Sykaras, N.; Iacopino, A.M.; Marker, V.A.; Triplett, R.G.; Woody, R.D. Implant materials, designs, and surface topographies: Their effect on osseointegration. A literature review. Int. J. Oral Maxillofac. Implant. 2000, 15, 675–690.
- Steinemann, S. Titanium–The material of choice? Periodontol 2000 1998, 17, 7–21.
- Huang, Y.S.; McGowan, T.; Lee, R.; Ivanovski, S. 7.23 Dental Implants: Biomaterial Properties Influencing Osseointegration. In Comprehensive Biomaterials II; Elsevier: Amsterdam, The Netherlands, 2017; Volume 7, pp. 444–466. ISBN 9780081006924.
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontology 2000 2017, 73, 22–40.
- Sharma, A.; Waddell, J.N.; Li, K.C.; Sharma, L.A.; Prior, D.J.; Duncan, W.J. Is titanium–zirconium alloy a better alternative to pure titanium for oral implant? Composition, mechanical properties, and microstructure analysis. Saudi Dent. J. 2021, 33, 546–553.
- Contaldo, M.; De Rosa, A.; Nucci, L.; Ballini, A.; Malacrinò, D.; La Noce, M.; Inchingolo, F.; Xhajanka, E.; Ferati, K.; Bexheti-Ferati, A.; et al. Titanium Functionalized with Polylysine Homopolymers: In Vitro Enhancement of Cells Growth. Materials 2021, 14, 3735.
- Yin, L.; Nakanishi, Y.; Alao, A.R.; Song, X.F.; Abduo, J.; Zhang, Y. A Review of Engineered Zirconia Surfaces in Biomedical Applications. In Procedia CIRP; Elsevier B.V.: Amsterdam, The Netherlands, 2017; Volume 65, pp. 284–290.
- Webber, L.P.; Chan, H.-L.; Wang, H.-L. Will Zirconia Implants Replace Titanium Implants? Appl. Sci. 2021, 11, 6776.
- Sivaraman, K.; Chopra, A.; Narayan, A.I.; Balakrishnan, D. Is zirconia a viable alternative to titanium for oral implant? A critical review. J. Prosthodont. Res. 2018, 62, 121–133.
- Chopra, D.; Jayasree, A.; Guo, T.; Gulati, K.; Ivanovski, S. Advancing dental implants: Bioactive and therapeutic modifications of zirconia. Bioact. Mater. 2021, 13, 161–178.
- Hafezeqoran, A.; Koodaryan, R. Effect of Zirconia Dental Implant Surfaces on Bone Integration: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2017, 2017, 9246721.
- Assal, P.A. The osseointegration of zirconia dental implants. Schweiz Monatsschr. Zahnmed. 2013, 123, 644–654.
- Cooper, L.F. A role for surface topography in creating and maintaining bone at titanium endosseous implants. J. Prosthet. Dent. 2000, 84, 522–534.
- Rompen, E.; Domken, O.; Degidi, M.; Pontes, A.E.F.; Piattelli, A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: A literature review. Clin. Oral Implant. Res. 2006, 17 (Suppl. S2), 55–67.
- Accioni, F.; Vázquez, J.; Merinero, M.; Begines, B.; Alcudia, A. Latest Trends in Surface Modification for Dental Implantology: Innovative Developments and Analytical Applications. Pharmaceutics 2022, 14, 455.
- Albrektsson, T.; Wennerberg, A. The Impact of Oral Implants-Past and Future, 1966–2042. J. Can. Dent. Assoc. 2005, 71, 327.
- Buser, D.; Schenk, R.K.; Steinemann, S.; Fiorellini, J.P.; Fox, C.H.; Stich, H. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 1991, 25, 889–902.
- Zhu, X.; Chen, J.; Scheideler, L.; Altebaeumer, T.; Geis-Gerstorfer, J.; Kern, D. Cellular reactions of osteoblasts to micron- and sub-micron-scale porous structures of titanium surfaces. Cells Tissues Organs 2004, 178, 13–22.
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184.
- Ketabi, M.; DePorter, D. The effects of laser microgrooves on hard and soft tissue attachment to implant collar surfaces: A literature review and interpretation. Int. J. Periodontics Restor. Dent. 2013, 33, e145–e152.
- Taniguchi, Y.; Kakura, K.; Yamamoto, K.; Kido, H.; Yamazaki, J. Accelerated Osteogenic Differentiation and Bone Formation on Zirconia with Surface Grooves Created with Fiber Laser Irradiation. Clin. Implant Dent. Relat. Res. 2015, 18, 883–894.
- Cervino, G.; Meto, A.; Fiorillo, L.; Odorici, A.; Meto, A.; D’Amico, C.; Oteri, G.; Cicciù, M. Surface Treatment of the Dental Implant with Hyaluronic Acid: An Overview of Recent Data. Int. J. Environ. Res. Public Health 2021, 18, 4670.
- von Wilmowsky, C.; Moest, T.; Nkenke, E.; Stelzle, F.; Schlegel, K.A. Implants in bone: Part I. A current overview about tissue response, surface modifications and future perspectives. Oral Maxillofac. Surg. 2014, 18, 243–257.
- Ventre, M.; Natale, C.F.; Rianna, C.; Netti, P.A. Topographic cell instructive patterns to control cell adhesion, polarization and migration. J. R. Soc. Interface 2014, 11, 20140687.
- Hotchkiss, K.M.; Sowers, K.T.; Olivares-Navarrete, R. Novel in vitro comparative model of osteogenic and inflammatory cell response to dental implants. Dent. Mater. 2019, 35, 176–184.
- Ponche, A.; Bigerelle, M.; Anselme, K. Relative influence of surface topography and surface chemistry on cell response to bone implant materials. Part 1: Physico-chemical effects. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2010, 224, 1471–1486.
- Santos, P.M.D.; Julio, E.N.B.S. A state-of-the-art review on roughness quantification methods for concrete surfaces. Constr. Build. Mater. 2013, 38, 912–923.
- Chen, S.; Feng, R.; Zhang, C.; Zhang, Y. Surface roughness measurement method based on multi-parameter modeling learning. Measurement 2018, 129, 664–676.
- Becker, S.T.; Beck-Broichsitter, B.E.; Rossmann, C.M.; Behrens, E.; Jochens, A.; Wiltfang, J. Long-term Survival of Straumann Dental Implants with TPS Surfaces: A Retrospective Study with a Follow-up of 12 to 23 Years. Clin. Implant Dent. Relat. Res. 2015, 18, 480–488.
- Åstrand, P.; Anzén, B.; Karlsson, U.; Sahlholm, S.; Svardstrom, P.; Hellem, S. Nonsubmerged Implants in the Treatment of the Edentulous Upper Jaw: A Prospective Clinical and Radiographic Study of ITI Implants—Results after 1 Year. Clin. Implant. Dent Relat. Res. 2000, 2, 166–174.
- Anselme, K.; Bigerelle, M.; Noel, B.; Dufresne, E.; Judas, D.; Iost, A.; Hardouin, P. Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. 2000, 49, 155–166.
- Blank, E.; Grischke, J.; Winkel, A.; Eberhard, J.; Kommerein, N.; Doll, K.; Yang, I.; Stiesch, M. Evaluation of biofilm colonization on multi-part dental implants in a rat model. BMC Oral Health 2021, 21, 313.
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918.
- Liao, H.; Fatash, B.; Li, J. Stability of hydroxyapatite-coatings on titanium oral implants (IMZ) 2 retrieved cases. Clin. Oral Implant. Res. 1997, 8, 68–72.
- Gittens, R.A.; Olivares-Navarrete, R.; Cheng, A.; Anderson, D.M.; McLachlan, T.; Stephan, I.; Geis-Gerstofer, J.; Sandhage, K.H.; Fedorov, A.G.; Rupp, F.; et al. The roles of titanium surface mi-cro/nanotopography and wettability on the differential response of human osteoblast lineage cells. Acta Biomater. 2013, 9, 6268–6277.
- Lellouche, J.; Friedman, A.; Gedanken, A.; Banin, E. Antibacterial and antibiofilm properties of yttrium fluoride nanoparticles. Int. J. Nanomed. 2012, 7, 5611–5624.
- Wyszogrodzka, G.; Marszałek, B.; Gil, B.; Dorozyński, P. Metal-organic frameworks: Mechanisms of antibacterial action and po-tential applications. Drug Discov. Today 2016, 21, 1009–1018.
- Mondal, D.; Nguyen, L.; Oh, I.-H.; Lee, B.-T. Microstructure and biocompatibility of composite biomaterials fabricated from titanium and tricalcium phosphate by spark plasma sintering. J. Biomed. Mater. Res. Part A 2012, 101A, 1489–1501.
- Kim, H.W.; Georgiou, G.; Knowles, J.C.; Koh, Y.H.; Kim, H.E. Calcium phosphates and glass composite coatings on zirconia for en-hanced biocompatibility. Biomaterials 2004, 25, 4203–4213.
- Yazdani, J.; Ahmadian, E.; Sharifi, S.; Shahi, S.; Dizaj, S.M. A short view on nanohydroxyapatite as coating of dental implants. Biomed. Pharmacother. 2018, 105, 553–557.
- Barrè Re, F.; van der Valk, C.M.; Meijer, G.; Dalmeijer, R.A.J.; de Groot, K.; Layrolle, P. Osteointegration of Biomimetic Apatite Coating Applied onto Dense and Porous Metal Implants in Femurs of Goats. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 67, 655–665.
- Habibovic, P.; Li, J.; van der Valk, C.M.; Meijer, G.; Layrolle, P.; van Blitterswijk, C.; de Groot, K. Biological performance of uncoated and octacalcium phosphate-coated Ti6Al4V. Biomaterials 2005, 26, 23–36.
- Rocci, A.; Rocci, M.; Rocci, C.; Scoccia, A.; Gargari, M.; Martignoni, M.; Gottlow, J.; Sennerby, L. Immediate loading of Brånemark system TiUnite and machined-surface implants in the posterior mandible, part II: A randomized open-ended 9-year follow-up clinical trial. Int. J. Oral Maxillofac. Implant. 2013, 28, 891–895.
- Traini, T.; Murmura, G.; Sinjari, B.; Perfetti, G.; Scarano, A.; D’Arcangelo, C.; Caputi, S. The Surface Anodization of Titanium Dental Implants Improves Blood Clot Formation Followed by Osseointegration. Coatings 2018, 8, 252.
- Shalabi, M.M.; Gortemaker, A.; Van’t Hof, M.V.; Jansen, J.A.; Creugers, N.H.J. Implant Surface Roughness and Bone Healing: A Systematic Review. J. Dent. Res. 2006, 85, 496–500.
- Sreeharsha, T.; Sharan, S.; Chandra, P.K.; Badola, I.; Jabeen, N.S. Implant surface modifications: A review. Int. J. Appl. Dent. Sci. 2020, 6, 334–338.
- Al-Nawas, B.; Groetz, K.A.; Goetz, H.; Duschner, H.; Wagner, W. Comparative histomorphometry and resonance frequency analysis of implants with moderately rough surfaces in a loaded animal model. Clin. Oral Implant. Res. 2007, 19, 1–8.
- Cho, S.A.; Park, K.T. The removal torque of titanium screw inserted in rabbit tibia treated by dual acid etching. Biomaterials 2003, 24, 3611–3617.
- Grassi, S.; Piattelli, A.; De Figueiredo, L.C.; Feres, M.; De Melo, L.; Iezzi, G.; Alba, R.C.; Shibli, J.A. Histologic Evaluation of Early Human Bone Response to Different Implant Surfaces. J. Periodontol. 2006, 77, 1736–1743.
- Hirano, T.; Sasaki, H.; Honma, S.; Furuya, Y.; Miura, T.; Yajima, Y.; Yoshinari, M. Proliferation and osteogenic differentiation of human mesenchymal stem cells on zirconia and titanium with different surface topography. Dent. Mater. J. 2015, 34, 872–880.
- Kim, H.-K.; Woo, K.M.; Shon, W.-J.; Ahn, J.-S.; Cha, S.; Park, Y.-S. Comparison of peri-implant bone formation around injection-molded and machined surface zirconia implants in rabbit tibiae. Dent. Mater. J. 2015, 34, 508–515.
This entry is offline, you can click here to edit this entry!
