Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Health Care Sciences & Services
Bezold’s abscess is a deep neck abscess related to otomastoiditis. Due to the insidious clinical presentation, diagnosis can be extremely challenging, leading to delays in treatment and possible life-threatening complications. Bezold’s abscess is found at any age, with overt male prevalence among adults. The clinical presentation, as well as the causative pathogens, are strikingly heterogeneous. Otomastoiditis and cholesteatoma are major risk factors.
- Bezold’s abscess
- Bezold
- abscess
- MRI
1. Introduction
Bezold’s abscess is a potentially life-threatening laterocervical/deep neck abscess complicating the course of an otomastoiditis [1,2]. First described in 1885 by Friedrich von Bezold [3], Bezold’s abscess is the spread of an aggressive middle ear and mastoid infectious process, through an erosion at the digastric ridge, deep in the neck between the digastric and sternocleidomastoid muscles (Figure 1).
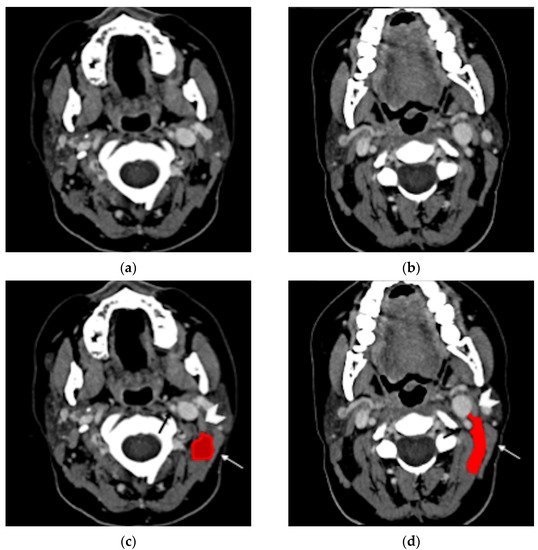
Figure 1. Normal anatomy, post-contrast axial CT (a,b). In the same images with annotations (c,d), the digastric muscle (white arrowhead) and the sternocleidomastoid muscle (white arrow) delimitate the posterior cervical space (red). The carotid artery can be identified nearby (black arrow).
Despite the high incidence of acute otitis media, Bezold’s abscesses are rare, especially in the pediatric population [4]. In a recent study considering around 5 million visits for acute otitis media, no patient presented such a complication [5]. However, Bezold’s abscesses are often underreported: over the last 20 years, at least four cases have been observed in the Neuroradiology Unit of Padova University Hospital. In addition, because of the high risk for life-threatening intracranial, neck, or even mediastinal complications [6], Bezold’s abscess requires early diagnosis, treatment, and surgery [7].
2. Epidemiologic, Imaging, and Clinical Issues in Bezold’s Abscess
2.1. Epidemiology
Bezold’s abscess is rare but can be found at any age. Its occurrence in the first five years of life has been considered anecdotal, due to the lack of pneumatization of the mastoid cells and the consequently thicker mastoid walls [8] that hamper the spread of the infection through this path. However, children under five represent nearly 7% of reported patients; this could be explained by a mere publication bias due to the well-known rarity and atypical presentation in the first years of life or by higher frequency of mastoiditis in children.
2.2. Pathogenesis
Bezold’s abscess typically complicates the course of chronic/recurrent otomastoiditis, even though few exceptions have been reported (acute otomastoiditis, external otitis, os tympanicum cholesteatoma). This specific type of abscess accounts for 6% of otogenic abscesses [11]. Indeed, otitis media commonly spreads to the mastoid. Subsequent inflammatory hypertrophy of the mucosa of aditus ad antrum might block the suppurative process into the mastoid cells, giving origin to coalescent mastoiditis [12].
According to the currently leading hypothesis, the retained purulent collection can lead to erosion of the cortical bone in a locus minoris resistentiae, the digastric groove, forcing the drainage of the pus into the neck (Bezold’s abscess) [3]. This hypothesis is supported by the common detection of cholesteatomas in Bezold’s abscess (40%).
Cholesteatomas are slowly growing masses usually appearing in the middle ear, occupying the Prussak space, expanding upwards and displacing the ossicles [13]. When a middle ear infection occurs, its growth pattern might block the path towards the external acoustic meatus [14] facilitating Bezold’s abscess formation. Notably, cholesteatoma may worsen the ventilation of the ear cavities promoting recurrent superinfections. In addition, bone erosion might be facilitated and is more conspicuous in the presence of cholesteatoma.
2.3. Clinical Presentation
Bezold’s abscess might be difficult to recognize, as its clinical presentation is highly heterogeneous, ranging from signs of neck tissue inflammation or fever to otorrhea or facial paralysis. Neck symptoms are the most reported (Table 2), even though publication bias cannot be excluded.
The evaluation through palpation is limited, as the purulent collection lies deep in the neck [7,8], where muscular and fascial planes constitute an anatomic barrier to the spread of the pus towards the surface.
However, involvement of the neck’s subcutaneous layers or skin is not rare in the literature (11/97 cases [8,18,19,20,21,22,23,24,25,26,27]), showing that the anatomical boundaries might be sometimes overcome, leading to unexpected diffusion pathways.
Bezold’s abscess is a consequence of a mastoiditis [3,8] accompanied by an history of acute (23%), chronic (19%), or recurrent (2%) otitis media; in five patients, a concomitant otitis externa was present [14,28,29,30]. Therefore, history of otomastoiditis and ipsilateral cervical swelling should raise the suspicion of Bezold’s abscess and proper radiological examinations should be applied. Notably, in the pediatric population, acute otitis media might show an asymptomatic or paucisymptomatic course, even without the presence of otorrhea [31,32].
Patient history, imaging, or surgical inspection usually reveal the presence of cholesteatoma, although in a few cases (10/39 cases) its existence has been detected by simple otoscopy [19,23,25,33,34,35,36,37,38,39]. In patients with neck pain or swelling, otoscopic evaluation is of utmost importance because it might show signs of otitis, raising suspicion of Bezold’s abscess (Table 2).
Table 2. Clinical presentation of Bezold’s abscess.
| Signs and Symptoms | Number of Patients (%) |
|---|---|
| Neck pain/tenderness | 57 (59%) |
| Neck swelling | 60 (62%) |
| Fever | 54 (56%) |
| Otorrhea | 54 (56%) |
| Hearing loss | 39 (40%) |
| Mixed | 7 (18%) |
| Conductive | 8 (21%) |
| Neurosensorial | 3 (8%) |
| Otitis | 42 (43%) |
| Acute | 22 (23%) |
| Chronic | 18 (19%) |
| Recurrent | 2 (2%) |
| Otalgia | 40 (41%) |
| Mastoid pain/tenderness | 36 (37%) |
| Mastoid swelling | 34 (35%) |
| Limitation in neck movements | 25 (26%) |
| Neck erythema | 23 (24%) |
| Neck stiffness/torticollis | 12 (12%) |
| Headache | 14 (14%) |
| Cranial nerves paralysis | |
| Facial nerve | 8 (8%) |
| Hypoglossal nerve | 1 (1%) |
| Abducens nerve | 1 (1%) |
| Abnormal tympanic membrane | 42 (43%) |
| Perforated | 14 (33%) |
| Inflamed | 10 (24%) |
| Retracted | 7 (17%) |
| Bulging | 5 (12%) |
| Thickened | 5 (12%) |
| Dull | 2 (5%) |
| Clinical history | |
| Cholesteatoma | 39 (40%) |
| Diabetes | 5 (5%) |
| Previous oto-mastoid surgery | 11 (11%) |
2.4. Differential Diagnosis
Differential diagnosis mainly includes lymphadenopathies, infected branchial cysts, temporal bone subperiosteal empyema, and other neck abscesses. The differential diagnoses are derived from available case reports investigating the main reasons for Bezold’s abscess diagnostic delay.
Bezold’s abscess might mimic a lymphadenopathy accompanying otitis [43]. Neck ultrasonography might help with recognizing reactive lymph nodes or identifying hypo/anechoic collections [44]. Infected branchial cysts might have a clinical and radiological presentation similar to Bezold’s abscess, but the mastoid is not involved, and bone erosion is never observed.
When dealing with suppurative processes originating from the mastoid bone, the diffusion pathway through the neck should be considered as this might be pivotal for subsequent evolution and for treatment planning. Suppurative processes from the mastoid might spread through bone erosion along three different paths: medial, lateral, or posteromedial [45]. Due to the complex anatomy of the several neck muscles and fasciae connected to the mastoid, the site of bone erosion will determine the subsequent diffusion path of the suppurative process that is eventually classified with different eponyms (Figure 3).
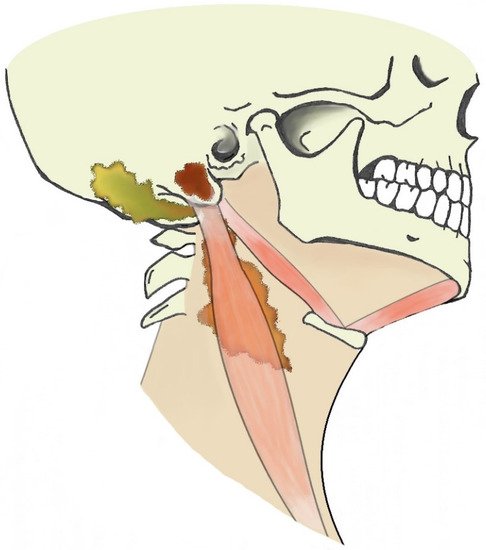
Figure 3. Illustration of extracranial otogenic abscesses. Bezold’s abscess (orange), Citelli’s abscess (yellow), subperiosteal empyema (brown).
A bone erosion medial to the mastoid allows the pus to spread to the posterior cervical and perivertebral spaces, deep into the sternocleidomastoid muscle (Bezold’s abscess); a posteromedial bone erosion can result in pus spreading posteriorly to the insertion of the digastric muscle and in the occipital region (Citelli’s abscess); a bone erosion lateral to the mastoid can give origin to a subperiosteal empyema that usually reaches the surface in the peri-mastoid subcutaneous spaces. However, this strict classification might not be appropriate in case of neck anatomy variants, erroneous identification of suppurative diffusion processes or coexistence of multiple paths.
2.5. Imaging Features
Ultrasonography can represent a valuable first-line diagnostic tool, especially with children, to exclude reactive lymph nodes and detect neck abscesses that appear as anechoic or hypoechoic inhomogeneous collections [44].
However, CT and MRI are the best tools in identifying abscesses and concomitant mastoiditis features and providing useful information for the surgical approach as simple neck abscess drainage might be insufficient for eradicating the infection and ultrasonography shows only the “tip of the iceberg” and not the source of infection [8]. In addition, a precise differentiation between Bezold’s abscess and other neck abscesses of otogenic origin might help the surgeon with localizing the bone defect while evaluating the mastoid [45].
2.6. CT
In the clinical suspicion of a Bezold’s abscess, temporal bone CT and contrast enhanced neck CT are the gold standard [7] for defining two main diagnostic aspects: (1) the site of mastoid bone erosion in the context of a mastoiditis and (2) the anatomical boundaries of the neck suppurative collection. Temporal bone CT typically shows signs of mastoiditis, such as opacification of the mastoid cells and erosion of the mastoid bone trabeculae. High resolution (slice thickness < 1 mm), coronal plane reconstructions, high frequency bone kernel and bone window are suggested to enable the detection of small bone interruptions at the mastoid tip, generally at the digastric groove (Figure 4).
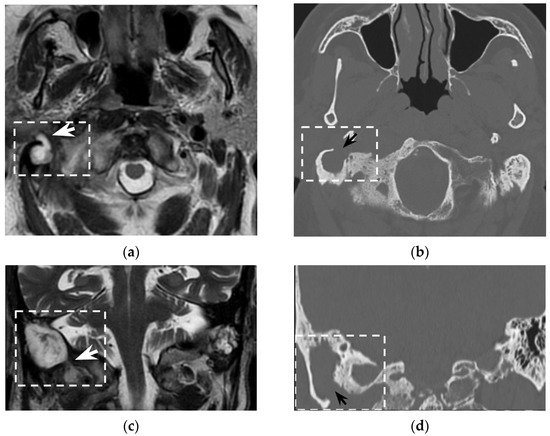
Figure 4. Eighty-seven-year-old man with history of external mycotic otitis. T2w MRI in axial (a) and coronal (c) planes show abnormal right mastoid (dotted rectangle) filled with hyperintense material that spreads into the neck (white arrows). Axial (b) and coronal (d) bone CT images show opacified right mastoid, absence of bone trabeculae, and a wide defect (black arrows) at the mastoid tip. Images were consistent with an infected cholesteatoma eroding the mastoid tip and spreading into the neck. The finding was confirmed at surgery.
However, despite its crucial pathogenic role for the suppurative path, bone defects in the mastoid tip have been precisely outlined only in around half of all literature reports (51/97 patients, 53%). Indeed, in 7/97 patients (7%) the mastoid tip was considered intact, revealing that the diagnosis of Bezold’s abscess can be achieved even without overt bone erosion, especially in younger children (3/7 patients were younger than 5 years old). The incomplete pneumatization of mastoid cells before the age of five is well known and is supposed to hinder the diffusion of the suppurative process across the thickened mastoid bone wall. Before the age of five, more destructive infectious processes or emissary vein bone canals likely allow Bezold’s abscess occurrence [8]. Temporal bone CT also allows for detection of intracranial bone erosions possibly leading to further life-threatening complications [8].
2.7. MRI
The role of MRI in the diagnosis of Bezold’s abscess is generally limited, being a “problem solving modality" used when intracranial complications or osseous disease are expected.
Bezold’s abscess entails a moderate risk of intracranial vascular (23/97) or further infectious (9/97) complications. MRI protocols should therefore include DWI and vascular imaging. DWI best recognizes suppurative collections (abscess, empyema, or even intraventricular debris) showing hyperintense signal on DWI with usually decreased apparent diffusion coefficient values (i.e., restriction of water molecule diffusion). DWI also detects cytotoxic oedema and intravascular thrombi helping in recognizing recent ischemic strokes or sinus venous thrombosis. Artery and venous intracranial MR-angiography can detect vessel occlusion consistent with thrombosis, thus confirming CT-angiography findings and allowing a less invasive follow-up. These sole sequences cover most intracranial complications of mastoiditis associated with Bezold’s abscess and are therefore of utmost importance for subsequent patient management.
2.8. Treatment
If Bezold’s abscess is present or suspected, broad spectrum antibiotic therapy with good cerebrospinal fluid penetration should be started, and appropriate imaging performed to evaluate location and size of the abscess collection. The routinely instituted empirical broad-spectrum antibiotic therapy should cover most of the Gram-positive and Gram-negative aerobic and anaerobic pathogens, given the incidence of polymicrobial infections (19/67 cases among patients with referred microbiological cultures). Early surgery is often mandatory to establish drainage of the middle ear (also through a myringotomy) and mastoid cells. It is necessary to carry out a sampling of the purulent material. The results of pathogen tests allow to replace as soon as possible the initially administered, broad-spectrum antibiotic with one to which the pathogen has (or the pathogens have) a known susceptibility [62,63]. If a deep neck fluid collection exists concurrently with a coalescent mastoiditis, a post-auricular incision is made, and a complete mastoidectomy should be performed in addition to the drainage of the deep neck abscess via a trans-cervical approach. After surgical drainage of the deep neck abscess collection, contrast-enhanced imaging control is recommended at 48–72 h, prior to removal of the suction drains.
This entry is adapted from the peer-reviewed paper 10.3390/tomography8020074
This entry is offline, you can click here to edit this entry!
