Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Electrochemistry
A typical type of Li–CO2 battery consists of a porous cathode, electrolyte (liquid, solid), and lithium metal anode.
- carbon tube-based cathode
- Li–CO2 battery
- reaction mechanism
1. Introduction
Global warming caused by greenhouse gases is an essential factor affecting the current environmental deterioration. The generation of greenhouse gases is inevitable based on the biomass (coal, oil, natural gas, etc.) combustion energy conversion method. Among them, carbon dioxide is the most important greenhouse gas, standing as the core issue that needs to be addressed to realize the currently advocated “low carbon environment” [1]. The solution strategy from “carbon peak” to “carbon neutralization” is divided into two aspects: one is the need to reduce carbon emissions, that is, to minimize energy storage and transformation based on “C”, and to gradually promote all types of renewable alternative energy in all walks of life; the second is the treatment of existing carbon dioxide with conventionally applied methods including chemical conversion, photocatalytic reduction, electrochemical reduction, and biological conversion [2]. The conversion efficiency of these methods has yet to be improved, while the biggest limitation is that the direct conversion of C in CO2 inevitably requires additional energy (resulting in extra “carbon emissions”), as C in CO2 is in the highest oxidation state [3]. The products obtained by these methods are carbon monoxide, methane, ethylene, formic acid, methanol, etc. The gaseous or liquid products are involved in compression, packaging, storage, transportation, and other steps before they are used as energy storage materials, which is bound to cause further energy loss [4]. Thus, the conventional CO2 conversion method is also a “high carbon” process.
In recent years, the research on lithium–air batteries has made significant progress, especially the developments focusing on the optimal catalyst selection and the structure of the carbon matrix composite cathode design [5,6,7,8]. Because of their high theoretical specific capacity (their theoretical specific capacity is 5–10 times that of lithium-ion batteries) [5,9,10], they are considered the ultimate devices for the energy storage of vehicle power batteries in the future. In the study of lithium–oxygen batteries, the effects of water vapor and CO2 have to be carefully considered. Research shows that the battery capacity under an O2/CO2 mixture is three times that of pure oxygen [11], but the stability decreases significantly. The study of the battery reaction process in a carbon dioxide atmosphere is an essential intermediate link to realize the real application of metal–air batteries in the future [6,12]. With the expansion of this research, Li–CO2 batteries have gradually developed into an independent research direction because this system can achieve potential applications in particular fields such as Mars (96% of carbon dioxide in the atmosphere with a low temperature) detection [13] and energy storage for submarines. In recent years, the number of related research papers published (Web of Science statistics) has increased year by year (Figure 1a), and the distribution of disciplines is shown in Figure 1b. Previous studies have shown that lithium–carbon dioxide batteries based on carbon-based cathode catalysis can achieve a stable cycle, and it is believed that the charging and discharging process is based on the following reaction: 4Li+ + 3CO2 + 4e−→2Li2CO3 + C (E0 = 2.80 V versus Li/Li+) [14,15,16]. This reaction has attracted wide attention in the fields of energy and the environment because it involves the fixation and transformation of CO2 in the electrochemical energy storage process. With the deepening of this research, the reversibility of the battery reaction has also sparked a controversial concept. At present, significant progress has been made in the study of the performance improvement of battery systems, such as the number of rechargeable cycles and the reduction in the overpotential. However, the research on the controllable preparation of optimized electrode materials and the corresponding reaction mechanism is still in its infancy.
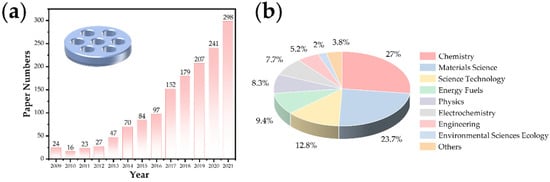
Figure 1. (a) The research progress and (b) distribution of disciplines of Li–CO2 batteries.
2. Structure and Reaction Mechanism of a Lithium–Carbon Dioxide Battery
2.1. The Structure of a Li–CO2 Battery
A typical type of Li–CO2 battery consists of a porous cathode, electrolyte (liquid, solid), and lithium metal anode [17]. The basic structure is shown in Figure 2, in the form of coin cells from different points of view.
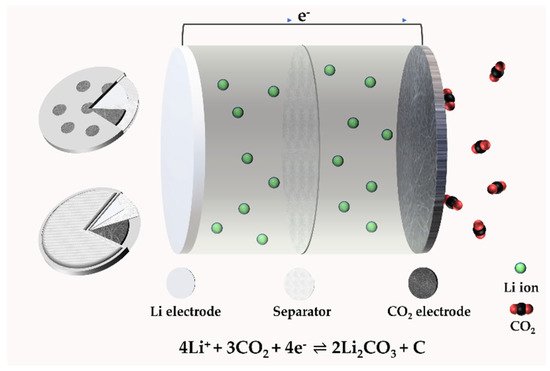
Figure 2. The structure of a Li–CO2 battery.
2.2. The Mechanism of a Li–CO2 Battery
The reaction process of Li–CO2 batteries is closely related to the electrode, electrolyte, and atmosphere environment. Studies have shown that lithium–carbon dioxide batteries cannot discharge in a pure CO2 atmosphere, and there must be a small amount of oxygen involved in the catalysis [11]. A much more critical problem is the lack of research evidence on the generation and decomposition process of discharge product C, and the lack of direct and powerful characterization test data. Zhou’s group reported in Joule that lithium–carbon dioxide batteries are rechargeable. Still, their charge and discharge processes are irreversible: lithium carbonate generated during discharge can be decomposed, but the generated carbon will not be decomposed but enriched on the electrode [18]. That is, when charging, the battery reaction is 2Li2CO3→2CO2 + O2 + 4Li+ + 4e−(E0 = 3.82 V versus Li/Li+). Based on this principle, an energy storage device can be designed, which can not only reduce the emission of CO2 but also use CO2 as the energy storage carrier. The gaseous CO2 is fixed into the solid C; that is, during the charging and discharging process, high specific energy storage and greenhouse gas treatment can be realized at the same time. The energy efficiency reaches 73.3% without pollution, which is of great significance to the solution of energy and environmental problems (Figure 3). Therefore, it is urgent to carry out proper mechanism research to confirm the reaction process of the system [19,20].
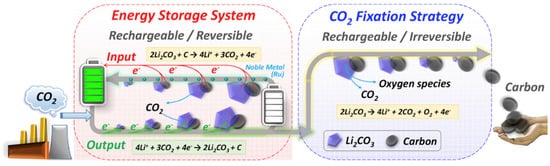
Figure 3. Schematic for the achievement of an energy storage system (reversible process) and a CO2 fixation strategy (irreversible process) via Li–CO2 electrochemistry technology. (Reprinted/adapted with permission from [18]. Copyright 2017 Elsevier).
2.3. The Application of DEMS in Electrode Interface Reaction
The macroscopic property of the electrode catalytic material interface lies in the chemical reaction. Since metal–air batteries involve the gas consumption and emission at the surface interface of catalytic materials, in recent years, the application of differential electrochemical mass spectrometry (DEMS) analysis based on gas detection in the field of lithium–oxygen batteries have realized the continuous measurement of gas, and in situ online analysis of the catalytic cathode interface reaction and possible side reactions [21,22]. The components of DEMS are shown in Figure 4a. In 2006, Bruce et al. added Li2O2 to the cathode of a lithium–air battery [23]. With the help of DEMS analysis, the results proved that O2 could be generated by oxidation during the charging process. The reaction mechanism of lithium–oxygen batteries was directly confirmed by experimental data. McCloskey et al. studied the formation and decomposition process of Li2O2 [21]. Peng et al. used nanoporous gold as a simulated cathode instead of a carbon-based cathode material [24]. The battery test results based on the DMSO electrolyte showed that no CO2 was detected. Based on the test of carbon-based materials, there will be apparent CO2 emission, indicating that lithium–air batteries with a carbon-based cathode have the possibility of decomposition in the use process, and the safety factor must be considered. The effect of nanocatalysts added to the cathode materials can also be evaluated by the DEMS test and the reaction measurement calculation, such as TiC and Mo2C, so as to speculate on the actual effect of the catalyst and analyze the stability of the electrode material [25]. The charge–discharge e−/reaction gas ratio can be calculated by Faraday’s law so as to speculate on the reaction path (Figure 4b). Another advantage of mass spectrometry is that it can be combined with isotope calibration methods, such as an isotope reaction gas or electrolyte, to track the intermediate products in the reaction process, and to effectively analyze the ion migration process and catalytic mechanism of the interface by in situ chemical testing methods [26].
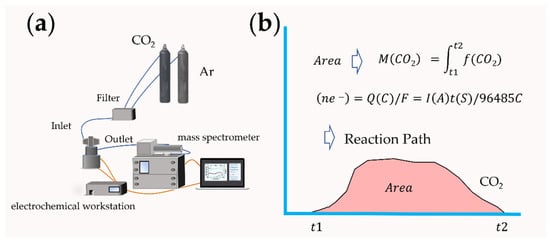
Figure 4. (a) Schematic for a DEMS system; (b) the process of predicting the reaction mechanism.
This entry is adapted from the peer-reviewed paper 10.3390/nano12122063
This entry is offline, you can click here to edit this entry!
