Immune checkpoint inhibitor treatment has shown revolutionary therapeutic effects in various carcinomas. However, immune-related adverse events (irAE) following the treatment can sometimes lead to treatment discontinuation. One such frequently encountered adverse event is immune-related colitis (irAE colitis). Corticosteroids (CS) are the first-line treatment for irAE colitis, but we often encounter CS-refractory or resistant cases. Application of multiple biologics has been proposed as a therapeutic drug to be administered after CS treatment; however, the efficacy and safety of biologics for patients with irAE colitis who do not respond to CS have not been established. This review summarizes the treatment regimens available for irAE colitis, focusing on the mechanism of action of corticosteroids, infliximab, vedolizumab, and other drugs.
- immune checkpoint inhibitor
- immune-related adverse events
- irAE colitis
1. Corticosteroids
| author | year | Pathogenic diseases | No. Case (CS Naïve: Need CS) |
Effects of CS on response rate or survival |
|---|---|---|---|---|
| Horvat et al. [33] | 2015 | melanoma | 195:103 | Systemic CS was not associated with OS or TTF |
| Weber et al. [34] | 2017 | melanoma | 462:114 | The ORR was 31.8% in the CS naïve group and 29.8% in the CS required group (p = 0.736). The median duration of response was 22.0 months in the CS naïve group and was not reached in the CS required group. |
| Skribek et al. [31] | 2020 | lung cancer | 104:31 | OS was 14.43 months in CS naïve group and not reached in CS required group (p = 0.38) |
2. Infliximab
3. Vedolizumab
4. Other Therapeutics
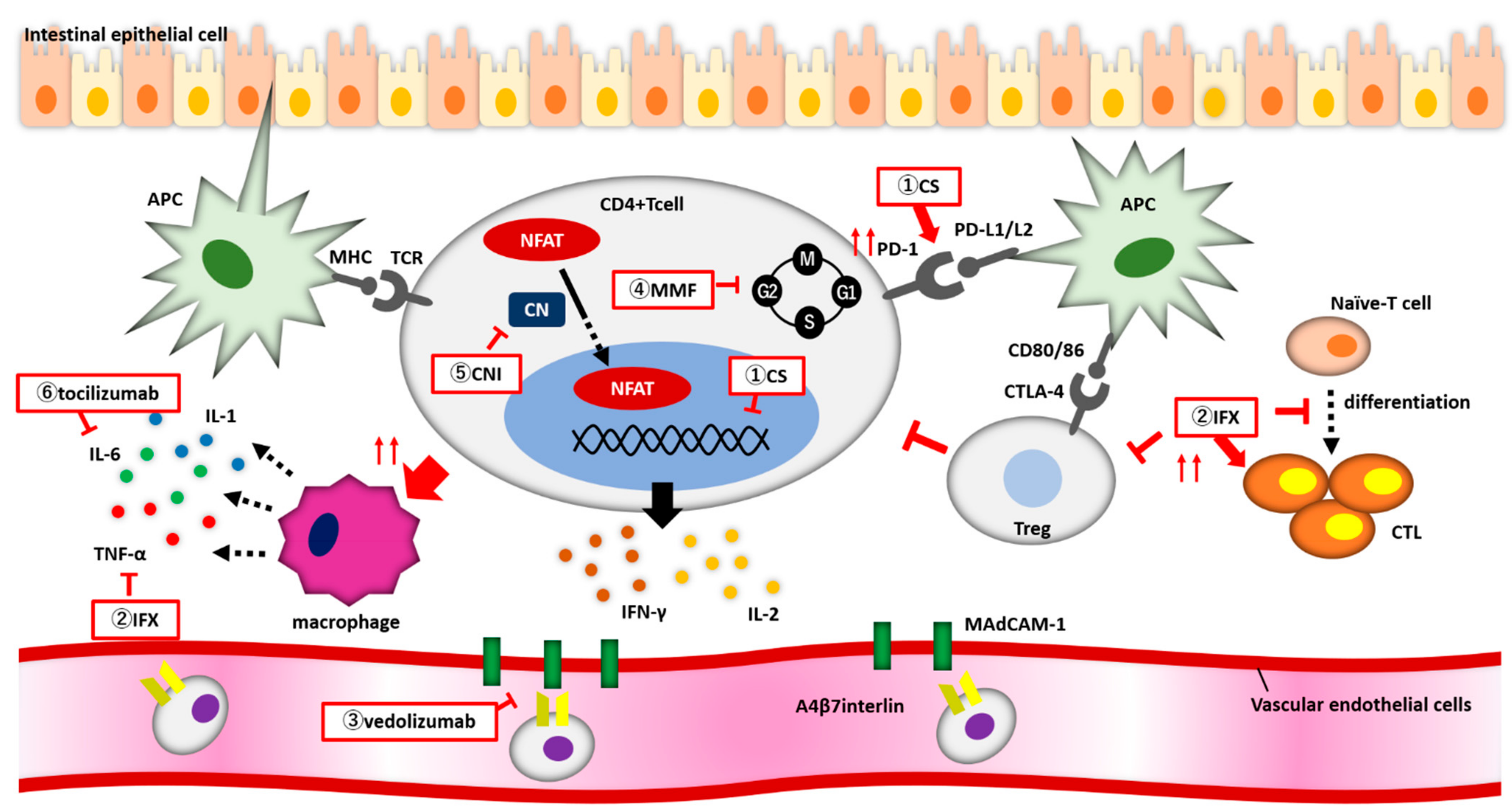
Figure 1. (1) Corticosteroids (CS) enhance PD-1 expression on the surface of CD4+ T cells. CS bind to the nuclear receptor of CD4+ T cell and suppress the release of inflammatory cytokines. (2) Infliximab enhances CTL activity, suppresses Treg function, and inhibits naive-T cell from differentiating into CTLs. (3) Vedolizumab inhibits the binding of α4β7 integrin to MadCAM-1 and blocks CD4+ T cells from migrating from blood vessels into the intestine. (4) MMF reversibly and specifically inhibits IMPDH, and lymphocytes arrest proliferation during the G1 to S phases of the cell cycle. (5) Calcineurin inhibitors block NFAT from migrating into the nucleus and reduce the expression of inflammatory cytokine genes.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10061334
References
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Andrews, S.; Armand, P.; Bhatia, S.; Budde, L.E.; Costa, L.; Davies, M.; Dunnington, D.; et al. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 230–241.
- Herold, M.J.; McPherson, K.G.; Reichardt, H.M. Glucocorticoids in T Cell Apoptosis and Function. Cell. Mol. Life Sci. 2006, 63, 60–72.
- Giles, A.J.; Hutchinson, M.N.D.; Sonnemann, H.M.; Jung, J.; Fecci, P.E.; Ratnam, N.M.; Zhang, W.; Song, H.; Bailey, R.; Davis, D.; et al. Dexamethasone-Induced Immunosuppression: Mechanisms and Implications for Immunotherapy. J. Immunother. Cancer 2018, 6, 51.
- Almawi, W.Y.; Beyhum, H.N.; Rahme, A.A.; Rieder, M.J. Regulation of Cytokine and Cytokine Receptor Expression by Glucocorticoids. J. Leukoc. Biol. 1996, 60, 563–572.
- Xing, K.; Gu, B.; Zhang, P.; Wu, X. Dexamethasone Enhances Programmed Cell Death 1 (PD-1) Expression during T Cell Activation: An Insight into the Optimum Application of Glucocorticoids in Anti-Cancer Therapy. BMC Immunol. 2015, 16, 39.
- Burla, J.; Bluemel, S.; Biedermann, L.; Barysch, M.J.; Dummer, R.; Levesque, M.P.; Gubler, C.; Morell, B.; Rogler, G.; Scharl, M. Retrospective Analysis of Treatment and Complications of Immune Checkpoint Inhibitor-Associated Colitis: Histological Ulcerations as Potential Predictor for a Steroid-Refractory Disease Course. Inflamm. Intest. Dis. 2020, 5, 109–116.
- Platanias, L.C. Mechanisms of type-I- and Type-II-Interferon-Mediated Signalling. Nat. Rev. Immunol. 2005, 5, 375–386.
- Luoma, A.M.; Suo, S.; Williams, H.L.; Sharova, T.; Sullivan, K.; Manos, M.; Bowling, P.; Hodi, F.S.; Rahma, O.; Sullivan, R.J.; et al. Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell 2020, 182, 655–671.
- Wang, T.; Zheng, N.; Luo, Q.; Jiang, L.; He, B.; Yuan, X.; Shen, L. Probiotics Lactobacillus Reuteri Abrogates Immune Checkpoint Blockade-Associated Colitis by Inhibiting Group 3 Innate Lymphoid Cells. Front. Immunol. 2019, 10, 1235.
- Sun, S.; Luo, L.; Liang, W.; Yin, Q.; Guo, J.; Rush, A.M.; Lv, Z.; Liang, Q.; Fischbach, M.A.; Sonnenburg, J.L.; et al. Bifidobacterium Alters the Gut Microbiota and Modulates the Functional Metabolism of T Regulatory Cells in the Context of Immune Checkpoint Blockade. Proc. Natl. Acad. Sci. USA 2020, 117, 2–8.
- Han, X.; Lee, A.; Huang, S.; Gao, J.; Spence, J.R.; Owyang, C. Lactobacillus Rhamnosus GG Prevents Epithelial Barrier Dysfunction Induced by Interferon-Gamma and Fecal Supernatants from Irritable Bowel Syndrome Patients in Human Intestinal Enteroids and Colonoids. Gut Microbes 2019, 10, 59–76.
- Mendoza, T.R.; Dueck, A.C.; Bennett, A.V.; Mitchell, S.A.; Reeve, B.B.; Atkinson, T.M.; Li, Y.; Castro, K.M.; Denicoff, A.; Rogak, L.J.; et al. Evaluation of Different Recall Periods for the US National Cancer Institute’s PRO-CTCAE. Clin. Trials 2017, 14, 255–263.
- Beck, K.E.; Blansfield, J.A.; Tran, K.Q.; Feldman, A.L.; Hughes, M.S.; Royal, R.E.; Kammula, U.S.; Topalian, S.L.; Sherry, R.M.; Kleiner, D.; et al. Enterocolitis in Patients with Cancer after Antibody Blockade of Cytotoxic T-Lymphocyte-Associated Antigen 4. J. Clin. Oncol. 2006, 24, 2283–2289.
- Geukes Foppen, M.H.; Rozeman, E.A.; van Wilpe, S.; Postma, C.; Snaebjornsson, P.; van Thienen, J.V.; van Leerdam, M.E.; van den Heuvel, M.; Blank, C.U.; van Dieren, J.; et al. Immune Checkpoint Inhibition-Related Colitis: Symptoms, Endoscopic Features, Histology and Response to Management. ESMO Open 2018, 3, e000278.
- Wang, D.Y.; Mooradian, M.J.; Kim, D.; Shah, N.J.; Fenton, S.E.; Conry, R.M.; Mehta, R.; Silk, A.W.; Zhou, A.; Compton, M.L.; et al. Clinical Characterization of Colitis Arising from Anti-PD-1 Based Therapy. Oncoimmunology 2019, 8, e1524695.
- Wang, Y.; Abu-Sbeih, H.; Mao, E.; Ali, N.; Qiao, W.; Trinh, V.A.; Zobniw, C.; Johnson, D.H.; Samdani, R.; Lum, P.; et al. Endoscopic and Histologic Features of Immune Checkpoint Inhibitor-Related Colitis. Inflamm. Bowel Dis. 2018, 24, 1695–1705.
- Prieux-Klotz, C.; Dior, M.; Damotte, D.; Dreanic, J.; Brieau, B.; Brezault, C.; Abitbol, V.; Chaussade, S.; Coriat, R. Immune Checkpoint Inhibitor-Induced Colitis: Diagnosis and Management. Target. Oncol. 2017, 12, 301–308.
- Yanai, S.; Nakamura, S.; Matsumoto, T. Nivolumab-Induced Colitis Treated by Infliximab. Clin. Gastroenterol. Hepatol. 2017, 15, e80–e81.
- Chen, J.H.; Pezhouh, M.K.; Lauwers, G.Y.; Masia, R. Histopathologic Features of Colitis Due to Immunotherapy with Anti-PD-1 Antibodies. Am. J. Surg. Pathol. 2017, 41, 643–654.
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768.
- Jain, A.; Lipson, E.J.; Sharfman, W.H.; Brant, S.R.; Lazarev, M.G. Colonic Ulcerations May Predict Steroid-Refractory Course in Patients with Ipilimumab-Mediated Enterocolitis. World J. Gastroenterol. 2017, 23, 2023–2028.
- Luo, J.; Beattie, J.A.; Fuentes, P.; Rizvi, H.; Egger, J.V.; Kern, J.A.; Leung, D.Y.M.; Lacouture, M.E.; Kris, M.G.; Gambarin, M.; et al. Beyond Steroids: Immunosuppressants in Steroid-Refractory or Resistant Immune-Related Adverse Events. J. Thorac. Oncol. 2021, 16, 1759–1764.
- Abdel-Wahab, N.; Shah, M.; Suarez-Almazor, M.E. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. PLoS ONE 2016, 11, e0160221.
- Coutzac, C.; Adam, J.; Soularue, E.; Collins, M.; Racine, A.; Mussini, C.; Boselli, L.; Kamsukom, N.; Mateus, C.; Charrier, M.; et al. Colon Immune-Related Adverse Events: Anti-CTLA-4 and Anti-PD-1 Blockade Induce Distinct Immunopathological Entities. J. Crohn’s Colitis 2017, 11, 1238–1246.
- Yoshino, K.; Nakayama, T.; Ito, A.; Sato, E.; Kitano, S. Severe Colitis After PD-1 Blockade with Nivolumab in Advanced Melanoma Patients: Potential Role of Th1-Dominant Immune Response in Immune-Related Adverse Events: Two Case Reports. BMC Cancer 2019, 19, 1019.
- Correale, P.; Saladino, R.E.; Giannarelli, D.; Sergi, A.; Mazzei, M.A.; Bianco, G.; Giannicola, R.; Iuliano, E.; Forte, I.M.; Calandruccio, N.D.; et al. HLA Expression Correlates to the Risk of Immune Checkpoint Inhibitor-Induced Pneumonitis. Cells 2020, 9, 1964.
- d’Apolito, M.; Spagnuolo, R.; Siciliano, M.A.; Barbieri, V.; Cosco, C.; Fiorillo, L.; Cuomo, O.; Zuccalà, V.; Correale, P.; Pensabene, L.; et al. Autoimmune Colitis and Neutropenia in Adjuvant Anti-PD-1 Therapy for Malignant Melanoma: Efficacy of Vedolizumab, a Case Report. Ther. Adv. Chronic Dis. 2022, 13, 20406223211063024.
- Sakurai, T.; De Velasco, M.A.; Sakai, K.; Nagai, T.; Nishiyama, H.; Hashimoto, K.; Uemura, H.; Kawakami, H.; Nakagawa, K.; Ogata, H.; et al. Integrative Analysis of Gut Microbiome and Host Transcriptomes Reveals Associations between Treatment Outcomes and Immunotherapy-Induced Colitis. Mol. Oncol. 2021, 16, 1493–1507.
- Stone, M.L.; Forster, E.M. Use of Vedolizumab in Immune Checkpoint Inhibitor-Associated Enterocolitis. Inflamm. Bowel Dis. 2021, 27, e147.
- Horvat, T.Z.; Adel, N.G.; Dang, T.O.; Momtaz, P.; Postow, M.A.; Callahan, M.K.; Carvajal, R.D.; Dickson, M.A.; D’Angelo, S.P.; Woo, K.M.; et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients with Melanoma Treated with Ipilimumab at Memorial Sloan Kettering Cancer Center. J. Clin. Oncol. 2015, 33, 3193–3198.
- Skribek, M.; Rounis, K.; Afshar, S.; Grundberg, O.; Friesland, S.; Tsakonas, G.; Ekman, S.; De Petris, L. Effect of Corticosteroids on the Outcome of Patients with Advanced Non-Small Cell Lung Cancer Treated with Immune-Checkpoint Inhibitors. Eur. J. Cancer 2021, 145, 245–254.
- Faje, A.T.; Lawrence, D.; Flaherty, K.; Freedman, C.; Fadden, R.; Rubin, K.; Cohen, J.; Sullivan, R.J. High-Dose Glucocorticoids for the Treatment of Ipilimumab-Induced Hypophysitis Is Associated with Reduced Survival in Patients with Melanoma. Cancer 2018, 124, 3706–3714.
- Weber, J.S.; Hodi, F.S.; Wolchok, J.D.; Topalian, S.L.; Schadendorf, D.; Larkin, J.; Sznol, M.; Long, G.V.; Li, H.; Waxman, I.M.; et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients with Advanced Melanoma. J. Clin. Oncol. 2017, 35, 785–792.
- Badran, Y.R.; Cohen, J.V.; Brastianos, P.K.; Parikh, A.R.; Hong, T.S.; Dougan, M. Concurrent Therapy with Immune Checkpoint Inhibitors and TNFα Blockade in Patients with Gastrointestinal Immune-Related Adverse Events. J. Immunother. Cancer 2019, 7, 226.
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768.
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Andrews, S.; Armand, P.; Bhatia, S.; Budde, L.E.; Costa, L.; Davies, M.; Dunnington, D.; et al. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 230–241.
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., 3rd; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing Toxicities Associated with Immune Checkpoint Inhibitors: Consensus Recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95.
- Yanai, S.; Nakamura, S.; Matsumoto, T. Nivolumab-Induced Colitis Treated by Infliximab. Clin. Gastroenterol. Hepatol. 2017, 15, e80–e81.
- Jain, A.; Lipson, E.J.; Sharfman, W.H.; Brant, S.R.; Lazarev, M.G. Colonic Ulcerations May Predict Steroid-Refractory Course in Patients with Ipilimumab-Mediated Enterocolitis. World J. Gastroenterol. 2017, 23, 2023–2028.
- Luo, J.; Beattie, J.A.; Fuentes, P.; Rizvi, H.; Egger, J.V.; Kern, J.A.; Leung, D.Y.M.; Lacouture, M.E.; Kris, M.G.; Gambarin, M.; et al. Beyond Steroids: Immunosuppressants in Steroid-Refractory or Resistant Immune-Related Adverse Events. J. Thorac. Oncol. 2021, 16, 1759–1764.
- Burla, J.; Bluemel, S.; Biedermann, L.; Barysch, M.J.; Dummer, R.; Levesque, M.P.; Gubler, C.; Morell, B.; Rogler, G.; Scharl, M. Retrospective Analysis of Treatment and Complications of Immune Checkpoint Inhibitor-Associated Colitis: Histological Ulcerations as Potential Predictor for a Steroid-Refractory Disease Course. Inflamm. Intest. Dis. 2020, 5, 109–116.
- Collins, M.; Michot, J.M.; Danlos, F.X.; Mussini, C.; Soularue, E.; Mateus, C.; Loirat, D.; Buisson, A.; Rosa, I.; Lambotte, O.; et al. Inflammatory Gastrointestinal Diseases Associated with PD-1 Blockade Antibodies. Ann. Oncol. 2017, 28, 2860–2865.
- Alexander, J.L.; Ibraheim, H.; Sheth, B.; Little, J.; Khan, M.S.; Richards, C.; Hunter, N.; Chauhan, D.; Ratnakumaran, R.; McHugh, K.; et al. Clinical Outcomes of Patients with Corticosteroid Refractory Immune Checkpoint Inhibitor-Induced Enterocolitis Treated with Infliximab. J. Immunother. Cancer 2021, 9, e002742.
- Lesage, C.; Longvert, C.; Prey, S.; Maanaoui, S.; Dréno, B.; Machet, L.; Zehou, O.; Kramkimel, N.; Jeudy, G.; Skowron, F.; et al. Incidence and Clinical Impact of Anti-TNFα Treatment of Severe Immune Checkpoint Inhibitor-Induced Colitis in Advanced Melanoma: The Mecolit Survey. J. Immunother. 2019, 42, 175–179.
- Miyahara, K.; Noda, T.; Ito, Y.; Hidaka, H.; Fujimoto, S.; Takedomi, H.; Akutagawa, T.; Sakata, Y.; Shimamura, T.; Tominaga, N.; et al. An Investigation of Nine Patients with Gastrointestinal Immune-Related Adverse Events Caused by Immune Checkpoint Inhibitors. Digestion 2020, 101, 60–65.
- Hillock, N.T.; Heard, S.; Kichenadasse, G.; Hill, C.L.; Andrews, J. Infliximab for Ipilimumab-Induced Colitis: A Series of 13 Patients. Asia Pac. J. Clin. Oncol. 2017, 13, e284–e290.
- Lesage, C.; Longvert, C.; Prey, S.; Maanaoui, S.; Dréno, B.; Machet, L.; Zehou, O.; Kramkimel, N.; Jeudy, G.; Skowron, F.; et al. Incidence and Clinical Impact of Anti-TNFα Treatment of Severe Immune Checkpoint Inhibitor-Induced Colitis in Advanced Melanoma: The Mecolit Survey. J. Immunother. 2019, 42, 175–179.
- Wang, Y.; Abu-Sbeih, H.; Mao, E.; Ali, N.; Ali, F.S.; Qiao, W.; Lum, P.; Raju, G.; Shuttlesworth, G.; Stroehlein, J.; et al. Immune-Checkpoint Inhibitor-Induced Diarrhea and Colitis in Patients with Advanced Malignancies: Retrospective Review at MD Anderson. J. Immunother. Cancer 2018, 6, 37.
- Verheijden, R.J.; May, A.M.; Blank, C.U.; Aarts, M.J.B.; van den Berkmortel, F.W.P.J.; van den Eertwegh, A.J.M.; de Groot, J.W.B.; Boers-Sonderen, M.J.; van der Hoeven, J.J.M.; Hospers, G.A.; et al. Association of Anti-TNF with Decreased Survival in Steroid Refractory Ipilimumab and Anti-PD1-Treated Patients in the Dutch Melanoma Treatment Registry. Clin. Cancer Res. 2020, 26, 2268–2274.
- Chen, A.Y.; Wolchok, J.D.; Bass, A.R. TNF in the Era of Immune Checkpoint Inhibitors: Friend or Foe? Nat. Rev. Rheumatol. 2021, 17, 213–223.
- Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Hanauer, S.; Colombel, J.F.; Sands, B.E.; Lukas, M.; Fedorak, R.N.; Lee, S.; Bressler, B.; et al. Vedolizumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2013, 369, 711–721.
- Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Hanauer, S.; Colombel, J.F.; Sands, B.E.; Lukas, M.; Fedorak, R.N.; Lee, S.; Bressler, B.; et al. Vedolizumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2013, 369, 711–721.
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.J.; Danese, S.; et al. Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2013, 369, 699–710.
- Hsieh, A.H.; Ferman, M.; Brown, M.P.; Andrews, J.M. Vedolizumab: A Novel Treatment for Ipilimumab-Induced Colitis. BMJ Case Rep. 2016, 2016, bcr2016216641.
- Kunogi, Y.; Tominaga, K.; Abe, K.; Kanazawa, M.; Tanaka, T.; Watanabe, S.; Kondo, M.; Kanamori, A.; Iijima, M.; Goda, K.; et al. Refractory Immune Checkpoint Inhibitor-Induced Colitis Improved by Tacrolimus: A Case Report. Healthcare 2021, 9, 418.
- Quandt, D.; Jasinski-Bergner, S.; Müller, U.; Schulze, B.; Seliger, B. Synergistic Effects of IL-4 and TNFα on the Induction of B7-H1 in Renal Cell Carcinoma Cells Inhibiting Allogeneic T Cell Proliferation. J. Transl. Med. 2014, 12, 151.
- Mir, R.; Shaw, H.M.; Nathan, P.D. Mycophenolate Mofetil Alongside High-Dose Corticosteroids: Optimizing the Management of Combination Immune Checkpoint Inhibitor-Induced Colitis. Melanoma Res. 2019, 29, 102–106.
- Iyoda, T.; Kurita, N.; Takada, A.; Watanabe, H.; Ando, M. Resolution of Infliximab-Refractory Nivolumab-Induced Acute Severe Enterocolitis after Cyclosporine Treatment in a Patient with Non-Small Cell Lung Cancer. Am. J. Case Rep. 2018, 19, 360–364.
- Iyoda, T.; Kurita, N.; Takada, A.; Watanabe, H.; Ando, M. Resolution of Infliximab-Refractory Nivolumab-Induced Acute Severe Enterocolitis after Cyclosporine Treatment in a Patient with Non-Small Cell Lung Cancer. Am. J. Case Rep. 2018, 19, 360–364.
- Mir, R.; Shaw, H.M.; Nathan, P.D. Mycophenolate Mofetil Alongside High-Dose Corticosteroids: Optimizing the Management of Combination Immune Checkpoint Inhibitor-Induced Colitis. Melanoma Res. 2019, 29, 102–106.
- Matsuoka, K.; Kobayashi, T.; Ueno, F.; Matsui, T.; Hirai, F.; Inoue, N.; Kato, J.; Kobayashi, K.; Kobayashi, K.; Koganei, K.; et al. Evidence-Based Clinical Practice Guidelines for Inflammatory Bowel Disease. J. Gastroenterol. 2018, 53, 305–353.
- Powell, N.; Ibraheim, H.; Raine, T.; Speight, R.A.; Papa, S.; Brain, O.; Green, M.; Samaan, M.A.; Spain, L.; Yousaf, N.; et al. British Society of Gastroenterology Endorsed Guidance for the Management of Immune Checkpoint Inhibitor-Induced Enterocolitis. Lancet Gastroenterol. Hepatol. 2020, 5, 679–697.
- Powell, N.; Ibraheim, H.; Raine, T.; Speight, R.A.; Papa, S.; Brain, O.; Green, M.; Samaan, M.A.; Spain, L.; Yousaf, N.; et al. British Society of Gastroenterology Endorsed Guidance for the Management of Immune Checkpoint Inhibitor-Induced Enterocolitis. Lancet Gastroenterol. Hepatol. 2020, 5, 679–697.
- Gout, T.; Ostör, A.J.; Nisar, M.K. Lower Gastrointestinal Perforation in Rheumatoid Arthritis Patients Treated with Conventional DMARDs or Tocilizumab: A Systematic Literature Review. Clin. Rheumatol. 2011, 30, 1471–1474.
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The Pro- and Anti-Inflammatory Properties of the Cytokine Interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888.
- Kaiser, G.C.; Yan, F.; Polk, D.B. Mesalamine Blocks tumor necrosis factor growth inhibition and nuclear factor kB activation in mouse colonocytes. Gastroenterology 1999, 116, 602–609.
- Criscuoli, V.; Modesto, I.; Orland, A.; Cottone, M. Mesalazine for the treatment of inflammatory bowel disease. Expert Opin. Pharmacother. 2013, 14, 1669–1678.
- Iwamoto, M.; Kato, K.; Moriyama, M.; Yamaguchi, K.; Takahashi, S. Remission of ulcerative colitis flare-up induced by nivolmab. Int. J. Colorectal Dis. 2020, 35, 1791–1795.
