Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The term “elderly” corresponds to different ages in the literature, but it is defined by considerable comorbidity and heterogeneity. Cancer incidence, specifically colorectal cancer, is increased in older patients with IE and impacts its outcome. Diagnosis of infective endocarditis (IE) in elderly patients is challenging due to the atypical presentation of the disease and the lower performance of imaging studies. Enterococcal etiology is more frequent than in younger patients.
- infective endocarditis
- elderly
- diagnosis
1. Epidemiology: A Growing Problem
The estimated annual incidence of IE is 3 to 15 cases per 100,000 [1][2][3][4][5][6], with wide regional variations. Despite the decline of rheumatic heart disease, a formerly widespread predisposing condition [7], the incidence appears to be increasing, especially at the expense of the elderly [8]. There is debate about the last restriction for antibiotic prophylaxis in international guidelines in this trend [9][10]. Still, other epidemiological changes from the last decades with enough influence need to be addressed [1][8][11].
First, life expectancy is increasing in high-income countries, nowadays being more than 80 years [12]. Accordingly, in Western countries, patients with IE are significantly older, with up to one-third of patients older than 70 years old [8][13][14]. Indeed, age itself is a risk factor, as older patients are estimated to have a five-fold higher risk of endocarditis [15][16].
Additionally, multiple studies addressing specific characteristics of IE in the elderly have found a high rate of comorbidities in this population (Table 1) [17][18][19][20][21][22][23]. However, due to the lack of large multicentric cohorts [24] and the heterogeneity of this population, there is not enough evidence to support specific recommendations, although some have made attempts [11][13].
Table 1. Special characteristics of the epidemiology of EI in the elderly in age-focused studies.
| Most Frequent Comorbidities (%) | Nosocomial Rate (%) | Valvular Prosthesis (%) | Endovascular Device (%) | Mortality In-Hospital (%) | Mortality at 1 Year (%) | |
|---|---|---|---|---|---|---|
| Durante-Magoni et al. (2008) [22] n = 2759 (>70 y, n = 773; <70 y, n = 1985) |
Chronic illness (54.1) * Diabetes mellitus (22.4) * Cancer (14.9) * |
20.3 * | 26.5 * | ND | 25.8 * | ND |
| López-Wolf et al. (2011) [21] n = 618 (>79 y, n = 34; <79 y, n = 584) |
Chronic anemia (30.3) * Diabetes mellitus (24.2) * Immunodepression (12.1) * |
ND | 23.5 * | 11.3* | 20.6 * | ND |
| Bassetti et al. (2014) [18] n = 436 (>75 y, n = 137; <75 y, n = 299) |
Chronic heart failure (47.7) * Chronic renal failure (29.2) * Cancer (25) * |
27.7 * | 40.2 * | ND | 22.6 | ND |
| Oliver et al. (2017) [20] n = 454 (>80 y, n = 51; <80 y, n = 403) |
High blood pressure (58.8) * History of cancer (29.4) * CKD (27.5) * |
23.5 * | 41.2 * | 4 | 15.7 | 37.3 * |
| Armiñanzas et al. (2019) [17] n = 3120 (>80 y, n = 502; <80 y, n = 2618) |
Congestive heart failure (40.5) * Diabetes mellitus (30.7) * Coronary arterial disease (25.7) * |
30.7 | 26.3 * | 16.3 * | 34.7 * | 20.4 * |
| Menchi-Elanzi et al. (2020) [19] n = 72 (>80 y, n = 18; <80 y, n = 54) |
Heart disease (72.2) * Diabetes mellitus (27.8) History of cancer (5.6) |
ND | 38.9 * | 16.7 | 5.6 | ND |
| Kiriyama et al. (2021) [23] n = 20,667 (>80 y, n = 4990; <80 y, n = 15,677) |
High blood pressure (31.8) * Diabetes mellitus (15.9) * Atrial Fibrillation (14) * |
ND | 0.7 * | ND | 22.8 * | ND |
* This data showed a statistically significant difference compared with other age groups. ND: no data.
In this regard, not even the term “elderly” is equally applied in the literature, having been used by international guidelines for patients older than sixty [25][26], but more recently for older than eighty. Meanwhile, some preferred to use the term “very elderly” [19] for over eighty-year-old patients, while others have referred to the term “octogenarian” [20]. In any case, aging is not a uniform process, including many different profiles [11].
Some authors have tried to define these profiles and compared them between different age groups. For example, Oliver et al. show that patients over 80 years old have more comorbidities, among the most common being cancer, diabetes, and renal disease, along with higher Charlson index and EuroSCORE, compared with younger patients [19][20]. Chronic heart disease and high blood pressure also have a higher prevalence [17][18][19][22][23]. Compared to the predominance of male sex in IE incidence among younger patients [21][24], some studies have found similar proportions of men and women among octogenarians, which could be partially attributable to women’s higher life expectancy [19].
Due to cardiac morbidities and aging, the prevalence of cardiovascular implantable devices and prosthetic valves is higher, including transcatheter aortic valve implantation (TAVI), which are well-known risk factors for IE, with even worse prognosis [3][21]. Moreover, predisposing events such as surgery, instrumentation, and recurrent infections are more common, being at high risk for bloodstream invasion [21][27]. This frequent contact with health care implies a higher rate of nosocomial infections [13] and resistant pathogens [22].
The risk of hematological and abdominal cancer increases in the first three months after IE diagnosis and remains higher than expected for up to 12 months in the case of abdominal cancer [28]. In addition, the incidence of IE in the elderly (and not other infections) increases up to fivefold around the diagnosis of colorectal cancer (CRC) compared with the diagnosis of lung, breast, or prostate cancer, or an arbitrary date for individuals without cancer [29]. CRC (or preneoplastic lesions such as adenomas) can cause IE by allowing the translocation of bacteria [30]. Still, there are other possible explanations for this association: IE patients often receive a colonoscopy as a part of the diagnostic work-up (indicated routinely in the case of S. gallolyticus IE [25][31][32][33] and increasingly recommended in E. faecalis IE [34][35][36][37][38]), leading to higher CRC detection; colonoscopy could increase itself the risk of IE by disrupting the bowel mucosa and allowing translocation; they share common etiologic factors. Concomitant diagnosis increases non-cancer-specific mortality and can produce changes in cancer treatment based on the predicted survival of patients [29].
Comorbidities could play not only a pathogenic role [15][22] but also have a strong influence on the outcome as in treatment selection [39]. Some studies have shown this population to have higher 1-year mortality than younger cohorts, although not higher global mortality [20]. Despite the recommendations of international guidelines, age has been found to be the main factor involved in the restrictive use of surgery [39][40]. Non-performance of surgery [16][20], along with the Charlson Comorbidity index and nutritional and functional status, was independently associated with mortality [17].
2. Clinical Presentation: The Impact of Comorbidities and Nonspecificity
More than a century after the first description of IE, this disease remains notable for its diverse and nonspecific presentation (Table 2), which often leads to a late diagnosis [41]. Furthermore, the clinical presentation of this disease in elderly patients is considered atypical [19], with nonspecific constitutional signs and symptoms, such as lethargy, fatigue, malaise, anorexia, and weight loss, being the most frequent manifestations. Clinical presentation as delirium is characteristic in this age group and can sometimes be the only manifestation [15]. However, fever, the most common initial symptom in the general population, is frequently absent in elderly patients [42]. Additionally, the high prevalence of baseline murmurs in older adults makes this finding nonspecific unless the murmur is clearly new [41]. Both the absence of fever and new-onset heart murmurs contribute to the delayed diagnosis of IE [21] (Figure 1).
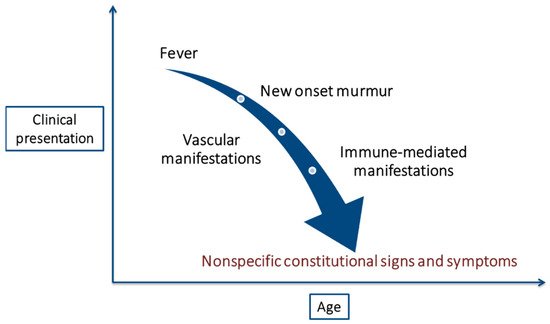
Figure 1. Changes in the clinical presentation of infective endocarditis with age.
| Clinical Presentation of IE | |
|---|---|
| Signs | Symptoms |
| New-onset heart murmur (50–85%) | Fever (90%) |
| Congestive heart failure (30%) | Chills |
| New conduction disturbances (2%) | Malaise |
| Disturbances in CNS (stroke, meningitis…) (14%) | Dyspnea |
| Peripheral septic abscesses or emboli | Anorexia |
| (renal, splenic, vertebral…) (5%) | Weight loss |
| Septic pulmonary emboli (6%) | Generalized weakness |
| Fever or sepsis of unknown origin | Back pain |
| Splinter hemorrhages (8%) | |
| Roth’s spots (8%) | |
| Acute kidney failure (23%) | |
| Anemia (Hb <10 g/dL) (20%) | |
Classic IE stigmas, surprisingly rare in the general population [41], are even less frequent in the elderly. Despite higher rates of mitral valve involvement, vascular manifestations of IE, such as embolic events, are less common in the elderly [42]. It could be explained by the widespread use of antiplatelets and anticoagulants among the older population. Immune-mediated manifestations are also less frequent, probably due to a less intense acute immune response. These two factors together (higher antiplatelet/anticoagulant use and weaker immune response) may help to explain the lower rate of vegetations found in this population group [22].
Therefore, we are faced with a vulnerable population group, in which the diagnosis is often more difficult due to the nonspecificity of its presentation and the higher prevalence of comorbidities compared to the general population [15].
3. Microbiology: From S. viridans to Enterococcal Etiology. Adequate Sample Collection Saves Lives
As previously stated, the diagnosis of IE in the elderly is rarely straightforward and is often delayed or overlooked because of atypical clinical presentation. Therefore, with clinical manifestations and imaging tests, the confirmatory diagnosis of IE must be based on microbiological findings [26].
Gram-positive cocci represent 80–90% of IE cases. The main microorganism isolated in young adults is Staphylococcus aureus, followed by the Streptococcus viridans group (includes S. anginosus, S. bovis/equinus, S. mitis, S. sanguinis, S. mutans, and S. salivarius, all commensals of the oral cavity and gastrointestinal tract) and Enterococcus spp. However, in patients 65 to 79 years old, Enterococcus spp. is detected more frequently. In octogenarians, S. viridans group and Enterococcus spp. are mainly isolated, with a few cases due to S. aureus (usually resistant to methicillin because of frequent exposure to medical attention). The isolation of coagulase-negative staphylococci is equally frequent in all age groups, although somewhat more frequent in patients older than 65 years and carriers of prosthetic devices or valves [19]. Streptococcus gallolyticus (formerly S. bovis) is noted for causing IE associated with underlying colonic pathology (ulcers, diverticular disease, and malignancy), which provides a gateway for bacteremia [31][32]. The association between E. faecalis IE and colonic pathology has also been described in the past few years [33][34][35][36][38].
Non-HACEK (Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, and Kingella) Gram-negative bacilli (GNB) constitute a rare cause of IE that has become more frequent than HACEK in recent years [44] due to the increase in age and comorbidity in the general population and the increase in enterobacterial bacteremia incidence [45][46][47]. Non-HACEK GNB Enterobacterales cases, predominantly caused by E. coli and more commonly associated with community acquisition, mitral valve involvement, genitourinary origin, and septic shock, were more frequent than non-HACEK GNB IE by non-fermentative GNB, principally P. aeruginosa, which is characterized by right valve involvement and health care association, predominantly venous catheter-related infection [44][48][49][50].
The cornerstone of the microbiological diagnosis of IE in all age groups continues to be blood culture (BC). Regardless of presentation, patients with unexplained persistent bacteremia or an unknown focus should be studied. The responsible organism can be recovered in 85–90% of patients by obtaining three blood samples collected at intervals of at least 30–60 min. It is not essential to perform the extraction coinciding with fever peaks since bacteremia in IE is constant. In positive blood cultures, the isolated microorganisms must be identified using highly reliable techniques (e.g., MALDI-TOF), as well as an antibiogram with determination of the MIC. Blood extractions through catheters should be avoided due to the difficulty of interpretation in cases of isolation of coagulase-negative staphylococci [25][26].
Approximately 10–15% of patients with IE have negative BC [51], making diagnosis even more complex and thus worsening the prognosis. Despite a lower risk clinical profile, these patients present higher in-hospital mortality than those with known etiology due to delayed diagnosis and, consequently, late initiation of antibiotic treatment [52]. The most common cause is the use of antibiotics before acquiring blood cultures (it is recommended to use blood culture bottles containing antimicrobial inhibitor resins). However, other possible explanations include infection by fastidious bacteria or fungi or alternative diagnoses such as non-bacterial thrombotic endocarditis (associated with hypercoagulable states or advanced neoplasms). Additional microbiological testing can identify a responsible organism in approximately two-thirds of patients. Thus, if blood cultures are negative at 5–7 days, serological tests for atypical organisms (Bartonella, Brucella, Coxiella, Chlamydia, Legionella, and Mycoplasma) should be considered [26][53].
Molecular techniques play a complementary role if embolic or valve material is available. The universal broad-spectrum polymerase chain reaction (PCR) for detecting 16s (bacteria) or 18S (fungi) rRNA is a sensitive technique that amplifies small amounts of microbial DNA and can identify the specific organism if combined with sequencing. This is particularly valuable in patients with previous exposure to antibiotics (since bacterial DNA often persists) and for non-culturable pathogens such as Tropheryma whipplei. However, false-positive results can arise due to sample contamination and PCR can also remain positive after eradicating viable bacteria (and, therefore, should not be used to guide the duration of therapy). New techniques that combine PCR with mass spectrometry hold promise for the direct characterization of bacteria in valve tissue [54].
4. Imaging Studies: Underperformance and Previous Valve Degeneration
Cardiac imaging techniques are one of the cornerstones in the diagnosis of IE. Echocardiography stands as the first line for the diagnosis and management of these patients [55]. Specifically, transesophageal echocardiography (TEE) provides additional value due to its higher resolution the better visualization of valvular structures. Other imaging techniques, such as CT, CMR, or PET, play a complementary role in specific cases [56].
Transthoracic echocardiography (TTE) must be performed promptly when IE is suspected. If TTE is suggestive of IE, TEE is indicated to better assess the extension of the infection and the valvular function. A TEE must also be performed in case of high clinical suspicion of IE if TEE is negative or non-diagnostic or in the case of prosthetic valves. Moreover, repeated TTE and/or TEE should be made 5–7 days after initial imaging if clinical suspicion of IE remains high [25][55][56]. Major diagnostic criteria of IE in echocardiography are vegetations, abscess or pseudoaneurysm, new valve regurgitation, and new dehiscence of a prosthetic valve. The characterization of these findings is also relevant for prognosis assessment and to guide therapeutic decisions. Thus, large vegetations, periannular complications, or severe valvular regurgitation pose a higher risk and support surgical indications in many cases. Three-dimensional TEE may provide additional value for this purpose, as it can better characterize vegetation size and perivalvular extension [57]. Follow-up echocardiography is recommended if a change in clinical condition occurs after initial diagnosis and to monitor response to medical therapy.
These recommendations apply to older patients, but some differences may be noted. López-Wolf et al. analyzed a cohort of 582 patients diagnosed with possible or definitive IE, comparing clinical and echocardiographic findings in three age groups: <65, 65–79, and ≥80 years [21]. TEE showed higher performance than TTE regardless of the age group. Vegetations were less likely to be detected in octogenarian patients than in their younger counterparts, both with TTE and TEE. TTE underperformance in the elderly may be related to a suboptimal acoustic window, whereas TEE can be attributed to degenerative changes in valvular structure. Valve degeneration, including fibrosis and calcification, is widespread in older patients, and it can be challenging to identify vegetation, even with TEE [21][24].
Multislice computed tomography (MSCT) provides high resolution to detect and characterize the perivalvular extension of IE (abscess, pseudoaneurysm, fistula) [58]. This technique may also be helpful in the case of prosthetic valve infection [59]. CT can diagnose IE complications such as embolization and its complications (infarcts, abscesses, etc.) [25]. It may also be used for coronary assessment before surgery in cases with a low risk of coronary artery disease [60]. MRI has a higher sensitivity than CT for detecting cerebral embolism [61]. Nuclear imaging techniques, such as SPECT and PET, are useful in borderline cases (i.e., possible IE using the Duke criteria), mainly if prosthetic material is affected [62].
This entry is adapted from the peer-reviewed paper 10.3390/jcdd9060192
References
- Bin Abdulhak, A.A.; Baddour, L.M.; Erwin, P.J.; Hoen, B.; Chu, V.H.; Mensah, G.A.; Tleyjeh, I.M. Global and Regional Burden of Infective Endocarditis, 1990-2010: A Systematic Review of the Literature. Glob. Heart 2014, 9, 131–143.
- Cahill, T.J.; Baddour, L.M.; Habib, G.; Hoen, B.; Salaun, E.; Pettersson, G.B.; Schäfers, H.J.; Prendergast, B.D. Challenges in Infective Endocarditis. J. Am. Coll. Cardiol. 2017, 69, 325–344.
- Ambrosioni, J.; Hernandez-Meneses, M.; Téllez, A.; Pericàs, J.; Falces, C.; Tolosana, J.; Vidal, B.; Almela, M.; Quintana, E.; Llopis, J.; et al. The Changing Epidemiology of Infective Endocarditis in the Twenty-First Century. Curr. Infect Dis. Rep. 2017, 19, 21.
- Wang, A.; Gaca, J.G.; Chu, V.H. Management Considerations in Infective Endocarditis: A Review. JAMA 2018, 320, 72–83.
- Pant, S.; Patel, N.J.; Deshmukh, A.; Golwala, H.; Patel, N.; Badheka, A.; Hirsch, G.A.; Mehta, J.L. Trends in Infective Endocarditis Incidence, Microbiology, and Valve Replacement in the United States from 2000 to 2011. J. Am. Coll. Cardiol. 2015, 65, 2070–2076.
- Hoen, B.; Duval, X. Clinical Practice. Infective Endocarditis. N. Engl. J. Med. 2013, 368, 1425–1433.
- Seckeler, M.D.; Hoke, T.R. The Worldwide Epidemiology of Acute Rheumatic Fever and Rheumatic Heart Disease. Clin. Epidemiol. 2011, 3, 67–84.
- Slipczuk, L.; Codolosa, J.N.; Davila, C.D.; Romero-Corral, A.; Yun, J.; Pressman, G.S.; Figueredo, V.M. Infective Endocarditis Epidemiology over Five Decades: A Systematic Review. PLoS ONE 2013, 8, e111564.
- Williams, M.L.; Doyle, M.P.; McNamara, N.; Tardo, D.; Mathew, M.; Robinson, B. Epidemiology of Infective Endocarditis before versus after Change of International Guidelines: A Systematic Review. Ther. Adv. Cardiovasc. Dis. 2021, 15, 17539447211002687.
- Duval, X.; Hoen, B. Prophylaxis for Infective Endocarditis: Let’s End the Debate. Lancet 2015, 385, 1164–1165.
- Chirillo, F. It Is Not How Old You Are, It Is How You Are Old: Need for Changes in the Management of Infective Endocarditis in the Elderly. Heart 2017, 103, 1562–1564.
- United Nations, Department of Economic and Social Affairs, Population Division. Available online: https://population.un.org/wpp/Download/Standard/Population/ (accessed on 30 March 2022).
- Forestier, E.; Fraisse, T.; Roubaud-Baudron, C.; Selton-Suty, C.; Pagani, L. Managing Infective Endocarditis in the Elderly: New Issues for an Old Disease. Clin. Interv. Aging 2016, 11, 1199–1206.
- Sy, R.W.; Kritharides, L. Health Care Exposure and Age in Infective Endocarditis: Results of a Contemporary Population-Based Profile of 1536 Patients in Australia. Eur. Heart J. 2010, 31, 1890–1897.
- Ursi, M.P.; Durante Mangoni, E.; Rajani, R.; Hancock, J.; Chambers, J.B.; Prendergast, B. Infective Endocarditis in the Elderly: Diagnostic and Treatment Options. Drugs Aging 2019, 36, 115–124.
- Di Salvo, G.; Thuny, F.; Rosenberg, V.; Pergola, V.; Belliard, O.; Derumeaux, G.; Cohen, A.; Iarussi, D.; Giorgi, R.; Casalta, J.P.; et al. Endocarditis in the Elderly: Clinical, Echocardiographic, and Prognostic Features. Eur. Heart J. 2003, 24, 1576–1583.
- Armiñanzas, C.; Fariñas-Alvarez, C.; Zarauza, J.; Muñoz, P.; González Ramallo, V.; Martínez Sellés, M.; Miró Meda, J.M.; Pericás, J.M.; Goenaga, M.Á.; Ojeda Burgos, G.; et al. Role of Age and Comorbidities in Mortality of Patients with Infective Endocarditis. Eur. J. Intern Med. 2019, 64, 63–71.
- Bassetti, M.; Venturini, S.; Crapis, M.; Ansaldi, F.; Orsi, A.; della Mattia, A.; Sinagra, G.; Pinamonti, B.; Rellini, G.; Moretti, V.; et al. Infective Endocarditis in Elderly: An Italian Prospective Multi-Center Observational Study. Int. J. Cardiol. 2014, 177, 636–638.
- Menchi-Elanzi, M.; Ramos-Rincón, J.M.; Merino-Lucas, E.; Reus-Bañuls, S.; Torrús-Tendero, D.; Clíment-Paya, V.; Boix, V.; Portilla-Sogorb, J. Infective Endocarditis in Elderly and Very Elderly Patients. Aging Clin. Exp. Res. 2020, 32, 1383–1388.
- Oliver, L.; Lavoute, C.; Giorgi, R.; Salaun, E.; Hubert, S.; Casalta, J.P.; Gouriet, F.; Renard, S.; Saby, L.; Avierinos, J.F.; et al. Infective Endocarditis in Octogenarians. Heart 2017, 103, 1602–1609.
- López-Wolf, D.; Vilacosta, I.; San Román, J.A.; Fernández, C.; Sarriá, C.; López, J.; Revilla, A.; Manchado, R. Endocarditis Infecciosa En Pacientes Octogenarios. Rev. Esp. de Cardiol. 2011, 64, 329–333.
- Durante-Mangoni, E. Current Features of Infective Endocarditis in Elderly Patients. Arch. Intern. Med. 2008, 168, 2095.
- Kiriyama, H.; Kaneko, H.; Itoh, H.; Kamon, T.; Morita, K.; Jo, T.; Fujiu, K.; Daimon, M.; Takeda, N.; Morita, H.; et al. Surgical Treatment for Infective Endocarditis in the Ageing Society: A Nationwide Retrospective Study in Japan. Open Heart 2021, 8, e001627.
- López, J.; Revilla, A.; Vilacosta, I.; Sevilla, T.; Villacorta, E.; Sarriá, C.; Pozo, E.; Rollán, M.J.; Gómez, I.; Mota, P.; et al. Age-Dependent Profile of Left-Sided Infective Endocarditis: A 3-Center Experience. Circulation 2010, 121, 892–897.
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; del Zotti, F.; Dulgheru, R.; el Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the Management of Infective Endocarditis. Eur. Heart J. 2015, 36, 3075–3123.
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals from the American Heart Association. Circulation 2015, 132, 1435–1486.
- Gregoratos, G. Infective Endocarditis in the Elderly: Diagnosis and Management. Am. J. Geriatr. Cardiol. 2003, 12, 183–189.
- Thomsen, R.W.; Farkas, D.K.; Friis, S.; Sværke, C.; Ording, A.G.; Norgaard, M.; Sorensen, H.T. Endocarditis and Risk of Cancer: A Danish Nationwide Cohort Study. Am. J. Med. 2013, 126, 58–67.
- García-Albéniz, X.; Hsu, J.; Lipsitch, M.; Logan, R.W.; Hernández-Díaz, S.; Hernán, M.A. Infective Endocarditis and Cancer in the Elderly. Eur. J. Epidemiol. 2016, 31, 41–49.
- Soler, A.P.; Miller, R.D.; Laughlin, K.V.; Carp, N.Z.; Klurfeld, D.M.; Mullin, J.M. Increased Tight Junctional Permeability Is Associated with the Development of Colon Cancer. Carcinogenesis 1999, 20, 1425–1431.
- Gupta, A.; Madani, R.; Mukhtar, H. Streptococcus Bovis Endocarditis, a Silent Sign for Colonic Tumour. Colorectal. Dis. 2010, 12, 164–171.
- Klein, R.S.; Recco, R.A.; Catalano, M.T.; Edberg, S.C.; Casey, J.I.; Steigbigel, N.H. Association of Streptococcus Bovis with Carcinoma of the Colon. N. Engl. J. Med. 1977, 297, 800–802.
- Corredoira, J.; García-País, M.J.; Coira, A.; Rabuñal, R.; García-Garrote, F.; Pita, J.; Rodríguez-Macías, A.; Blanco, M.; Lopez-Roses, L.; López-Álvarez, M.J.; et al. Differences between Endocarditis Caused by Streptococcus Bovis and Enterococcus Spp. and Their Association with Colorectal Cancer. Eur. J. Clin. Microbiol. Infect Dis. 2015, 34, 1657–1665.
- Khan, Z.; Siddiqui, N.; Saif, M.W. Enterococcus Faecalis Infective Endocarditis and Colorectal Carcinoma: Case of New Association Gaining Ground. Gastroenterol. Res. 2018, 11, 238–240.
- Silva, E.C.F.; Montalvão, C.R.; Bonafé, S. Infectious Endocarditis from Enterococcus Faecalis Associated with Tubular Adenoma of the Sigmoid Colon. Case Rep. Infect Dis. 2017, 2017, 3095031.
- Escolà-Vergé, L.; Peghin, M.; Givone, F.; Pérez-Rodríguez, M.T.; Suárez-Varela, M.; Meije, Y.; Abelenda, G.; Almirante, B.; Fernández-Hidalgo, N. Prevalence of Colorectal Disease in Enterococcus Faecalis Infective Endocarditis: Results of an Observational Multicenter Study. Rev. Esp. Cardiol. (Engl. Ed.) 2020, 73, 711–717.
- Pericàs, J.M.; Corredoira, J.; Moreno, A.; García-País, M.J.; Falces, C.; Rabuñal, R.; Mestres, C.A.; Alonso, M.P.; Marco, F.; Quintana, E.; et al. Relationship between Enterococcus Faecalis Infective Endocarditis and Colorectal Neoplasm: Preliminary Results from a Cohort of 154 Patients. Rev. Esp. Cardiol. (Engl. Ed.) 2017, 70, 451–458.
- Pericàs, J.M.; Ambrosioni, J.; Muñoz, P.; de Alarcón, A.; Kestler, M.; Mari-Hualde, A.; Moreno, A.; Goenaga, M.; Fariñas, M.C.; Rodríguez-Álvarez, R.; et al. Prevalence of Colorectal Neoplasms Among Patients With Enterococcus Faecalis Endocarditis in the GAMES Cohort (2008–2017). Mayo Clin. Proc. 2021, 96, 132–146.
- Selton-Suty, C.; Hoen, B.; Grentzinger, A.; Houplon, P.; Maignan, M.; Juillière, Y.; Danchin, N.; Canton, P.; Cherrier, F. Clinical and Bacteriological Characteristics of Infective Endocarditis in the Elderly. Heart 1997, 77, 260–263.
- Cox, L.; Kloseck, M.; Crilly, R.; McWilliam, C.; Diachun, L. Underrepresentation of Individuals 80 Years of Age and Older in Chronic Disease Clinical Practice Guidelines. Can. Fam. Physician 2011, 57, e263.
- Yang, E.; Frazee, B.W. Infective Endocarditis. Emerg. Med. Clin. N. Am 2018, 36, 645–663.
- Lemos, L.H.B.; da Silva, L.R.; Correa, M.G.; Golebiovski, W.; Weksler, C.; Garrido, R.Q.; Barbosa, G.F.; da Cruz Lamas, C. Infective Endocarditis in the Elderly: Distinct Characteristics. Arq. Bras. de Cardiol. 2021, 117, 775–781.
- Cahill, T.J.; Prendergast, B.D. Infective Endocarditis. Lancet 2016, 387, 882–893.
- Calderón Parra, J.; de Castro-Campos, D.; Muñoz García, P.; Olmedo Samperio, M.; Marín Arriaza, M.; de Alarcón, A.; Gutierrez-Carretero, E.; Fariñas Alvarez, M.C.; Miró Meda, J.M.; Goneaga Sanchez, M.Á.; et al. Non-HACEK Gram Negative Bacilli Endocarditis: Analysis of a National Prospective Cohort. Eur. J. Intern. Med. 2021, 92, 71–78.
- Diekema, D.J.; Hsueh, P.R.; Mendes, R.E.; Pfaller, M.A.; Rolston, K.V.; Sader, H.S.; Jones, R.N. The Microbiology of Bloodstream Infection: 20-Year Trends from the SENTRY Antimicrobial Surveillance Program. Antimicrob. Agents Chemother. 2019, 63, e00355-19.
- De Kraker, M.E.A.; Jarlier, V.; Monen, J.C.M.; Heuer, O.E.; van de Sande, N.; Grundmann, H. The Changing Epidemiology of Bacteraemias in Europe: Trends from the European Antimicrobial Resistance Surveillance System. Clin. Microbiol. Infect. 2013, 19, 860–868.
- Graff, L.R.; Franklin, K.K.; Witt, L.; Cohen, N.; Jacobs, R.A.; Tompkins, L.; Joseph Guglielmo, B. Antimicrobial Therapy of Gram-Negative Bacteremia at Two University-Affiliated Medical Centers. Am. J. Med. 2002, 112, 204–211.
- Morpeth, S.; Murdoch, D.; Cabell, C.H.; Karchmer, A.W.; Pappas, P.; Levine, D.; Nacinovich, F.; Tattevin, P.; Fernández-Hidalgo, N.; Dickerman, S.; et al. Non-HACEK Gram-Negative Bacillus Endocarditis. Ann. Intern Med. 2007, 147, 829–835.
- Falcone, M.; Tiseo, G.; Durante-Mangoni, E.; Ravasio, V.; Barbaro, F.; Ursi, M.P.; Pasticci, M.B.; Bassetti, M.; Grossi, P.; Venditti, M.; et al. Risk Factors and Outcomes of Endocarditis Due to Non-HACEK Gram-Negative Bacilli: Data from the Prospective Multicenter Italian Endocarditis Study Cohort. Antimicrob. Agents Chemother. 2018, 62, e02208-17.
- Loubet, P.; Lescure, F.X.; Lepage, L.; Kirsch, M.; Armand-Lefevre, L.; Bouadma, L.; Lariven, S.; Duval, X.; Yazdanpanah, Y.; Joly, V. Endocarditis Due to Gram-Negative Bacilli at a French Teaching Hospital over a 6-Year Period: Clinical Characteristics and Outcome. Infect Dis. (Lond.) 2015, 47, 889–895.
- Murdoch, D.R.; Corey, R.G.; Hoen, B.; Miró, M.; Fowler, V.G.; Bayer, A.S.; Karchmer, A.W.; Olaison, L.; Pappas, P.A.; Moreillon, P.; et al. Clinical Presentation, Etiology, and Outcome of Infective Endocarditis in the 21st Century: The International Collaboration on Endocarditis-Prospective Cohort Study. Arch. Intern. Med. 2009, 169, 463–473.
- Díez-Villanueva, P.; Muñoz, P.; Marín, M.; Bermejo, J.; de Alarcón González, A.; Fariñas, M.C.; Gutiérrez-Cuadra, M.; Pericás-Pulido, J.M.; Lepe, J.A.; Castelo, L.; et al. Infective Endocarditis: Absence of Microbiological Diagnosis Is an Independent Predictor of Inhospital Mortality. Int. J. Cardiol. 2016, 220, 162–165.
- Gould, F.K.; Denning, D.W.; Elliott, T.S.J.; Foweraker, J.; Perry, J.D.; Prendergast, B.D.; Sandoe, J.A.T.; Spry, M.J.; Watkin, R.W. Guidelines for the Diagnosis and Antibiotic Treatment of Endocarditis in Adults: A Report of the Working Party of the British Society for Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 2012, 67, 269–289.
- Peeters, B.; Herijgers, P.; Beuselinck, K.; Peetermans, W.E.; Herregods, M.C.; Desmet, S.; Lagrou, K. Comparison of PCR-Electrospray Ionization Mass Spectrometry with 16S RRNA PCR and Amplicon Sequencing for Detection of Bacteria in Excised Heart Valves. J. Clin. Microbiol. 2016, 54, 2825–2831.
- Habib, G.; Badano, L.; Tribouilloy, C.; Vilacosta, I.; Zamorano, J.L.; Galderisi, M.; Voigt, J.U.; Sicari, R.; Cosyns, B.; Fox, K.; et al. Recommendations for the Practice of Echocardiography in Infective Endocarditis. Eur. J. Echocardiogr. 2010, 11, 202–219.
- Bruun, N.E.; Habib, G.; Thuny, F.; Sogaard, P. Cardiac Imaging in Infectious Endocarditis. Eur. Heart J. 2014, 35, 624–632.
- Berdejo, J.; Shibayama, K.; Harada, K.; Tanaka, J.; Mihara, H.; Gurudevan, S.V.; Siegel, R.J.; Shiota, T. Evaluation of Vegetation Size and Its Relationship with Embolism in Infective Endocarditis: A Real-Time 3-Dimensional Transesophageal Echocardiography Study. Circ. Cardiovasc. Imaging 2014, 7, 149–154.
- Feuchtner, G.M.; Stolzmann, P.; Dichtl, W.; Schertler, T.; Bonatti, J.; Scheffel, H.; Mueller, S.; Plass, A.; Mueller, L.; Bartel, T.; et al. Multislice Computed Tomography in Infective Endocarditis: Comparison with Transesophageal Echocardiography and Intraoperative Findings. J. Am. Coll. Cardiol. 2009, 53, 436–444.
- Fagman, E.; Perrotta, S.; Bech-Hanssen, O.; Flinck, A.; Lamm, C.; Olaison, L.; Svensson, G. ECG-Gated Computed Tomography: A New Role for Patients with Suspected Aortic Prosthetic Valve Endocarditis. Eur. Radiol. 2012, 22, 2407–2414.
- Hekimian, G.; Kim, M.; Passefort, S.; Duval, X.; Wolff, M.; Leport, C.; Leplat, C.; Steg, G.; Iung, B.; Vahanian, A.; et al. Preoperative Use and Safety of Coronary Angiography for Acute Aortic Valve Infective Endocarditis. Heart 2010, 96, 696–700.
- Cooper, H.A.; Thompson, E.C.; Laureno, R.; Fuisz, A.; Mark, A.S.; Lin, M.; Goldstein, S.A. Subclinical Brain Embolization in Left-Sided Infective Endocarditis: Results from the Evaluation by MRI of the Brains of Patients with Left-Sided Intracardiac Solid Masses (EMBOLISM) Pilot Study. Circulation 2009, 120, 585–591.
- Saby, L.; Laas, O.; Habib, G.; Cammilleri, S.; Mancini, J.; Tessonnier, L.; Casalta, J.P.; Gouriet, F.; Riberi, A.; Avierinos, J.F.; et al. Positron Emission Tomography/Computed Tomography for Diagnosis of Prosthetic Valve Endocarditis: Increased Valvular 18F-Fluorodeoxyglucose Uptake as a Novel Major Criterion. J. Am. Coll. Cardiol. 2013, 61, 2374–2382.
This entry is offline, you can click here to edit this entry!
