Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Bladder pathologies, very common in the aged population, have a considerable negative impact on quality of life. Novel targets are needed to design drugs and combinations to treat diseases such as overactive bladder and bladder cancers. A promising new target is the ubiquitous Rho GTPase Rac1, frequently dysregulated and overexpressed in bladder pathologies.
- bladder cancer
- Rho GTPase
- bladder dysfunction
- Rac inhibitors
- metastasis
- overactive bladder
- Rac1 protein
1. Rac1 Structure and Function
Like many other GTPases, Rac1 switches between an inactive GDP-bound and an active GTP-bound state during signal transduction [32] (Figure 2c). The protein is involved in a wide range of cellular and physiological processes via a multiplicity of protein partners, among which a variety of guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) essential to control Rac1 activity [33]. In addition, a diversity of effector proteins can modulate Rac1 function, such as the serine-threonine kinases PAK1 (p21-activated kinase 1), MLK-1-3 (mixed-lineage kinases), p70 S6 kinase, CaMKII and many other kinases [20]. The local availability of GTP in cells plays a role in the control of Rac1 activity [34]. The expression and subcellular localization of Rac1 is also regulated at the post-transcriptional level via structural modifications, including phosphorylation, ubiquitination, adenylylation, and lipidation (prenylation, geranylgeranylation, palmitoylation) [35]. The lipid anchor is positioned in a hypervariable region, distant from the guanine nucleotide-binding domain, but contributing importantly to the interaction with effectors [36]. The protein is generally attached to the plasma membrane, but it can also be found in the nucleus and/or mitochondria [37]). At the membrane level, Rac1 can form nanoclusters acting as lipid-based signaling platforms [38].
The GDP/GTP loading status and cycling rate of Rac1 determine the protein activity. The nucleotide cycling process is impacted by the intrinsic conformational flexibility of the protein and the Mg2+ abundance [39]. The level of expression of the protein can vary significantly. An epigenetic downregulation of Rac1 has been reported in patients suffering from depression [40]. Conversely, Rac1 is often overexpressed and hyperactivated in cancers, notably in breast, colon, skin (melanoma), liver and lung cancers [31,41,42,43]. Moreover, Rac1 gain-of-function mutations have been identified in recent years, such as the two somatic mutations Rac1P29S and Rac1A159V, respectively detected in melanoma and in head-and-neck cancers [44,45,46], and occasionally observed in colon, thyroid, and lung cancers [47]. These variants represent fast cycling mutants that contribute to expand tumor phenotypes and confer resistance to targeted therapies. There exists also an alternatively spliced isoform designated Rac1b, with versatile functions, generally involved in tumor progression, but occasionally described as being engaged in the blockade of tumors [48,49]. To our knowledge, neither the fast-cycling oncogenic mutant enzymes nor the spliced variant Rac1b have been reported in bladder cancer or bladder pathologies.
2. Rac1 in Non-Cancerous Bladder Pathologies
2.1. Rac1 and Bacterial Infections of the Bladder
The ubiquitous Rho GTPase Rac1 plays key roles in the regulation of the cytoskeleton dynamic and cell motility in general. The protein is associated with the formation of protrusions at leading edge of migrating cells (lamellipodia, filopodia), whatever the cell type. As such, Rac1, actively participates to control cellular proliferation and cell mobility. It also plays a role in bacterial attachment to host cells and infections, notably in bladder infections caused by uropathogenic Escherichia coli [50]. Urinary tract infection (UTI) produced by uropathogenic E. coli (UPEC) promotes the sensitization of bladder afferent sensory neurons and the virulence factors produced by those bacteria contribute to the sensitization of bladder afferents in UTI [51]. The uroepithelial invasion by the bacteria occurs through lipid rafts, and Rac1 associated with caveolin-1 in those rafts is required for the bacterial invasion [52]. Rac1 activation enhances the accumulation of actin filaments at sites of bacterial entry (Figure 3). The use of bladder epithelial cells overexpressing constitutively activated Rac1, or conversely, cells with the dominant negative form, has clearly demonstrated that Rac1 activation is essential to the invasion of bladder epithelial cells by type 1 fimbriated E. coli. Moreover, the inhibition of Rac-1 activation via a Toll-like receptor 4 (TLR4)-mediated mechanism was found to suppress bacterial invasion [53]. In fact, bacterial lipopolysaccharides engage the TLR4/Rac1/Akt signaling pathway to enter cells and mediate the proliferation of vascular smooth muscle cells [54]. Once in the cells, the bacteria produce a toxin CNF1 (cytotoxic necrotizing factor type (1), which constitutively activates different Rho GTPases, including Rac1 critical to phagocytosis, to promote further infection [55]. The key role of Rac1 in the invasion of bladder epithelial cells by uropathogenic bacteria suggests that a negative regulation of Rac1 can be an option to reduce and combat infections of the urinary bladder. This can be achieved directly with Rac1-targeting small molecules (discussed below) or indirectly with compounds interfering with Rac1-mediated actin polymerization, as shown with the dietary flavonoid luteolin, for example [56].
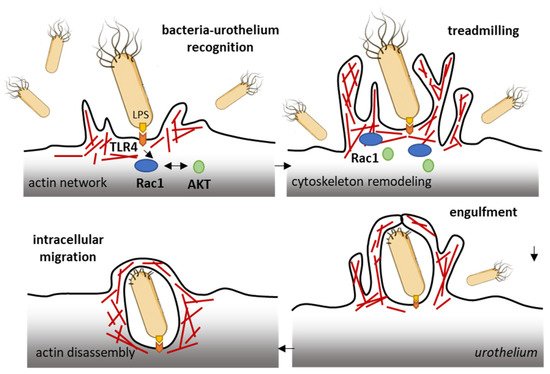
Figure 3. Rac1 plays a role in the invasion of bladder epithelial cells by type 1 fimbriated E. coli. Bacterial lipopolysaccharides activate the TLR4/Rac1/Akt signaling pathway to enter cells vascular smooth muscle cells and colonize the bladder tissue [54]. The Rac1 GTPase-mediated contributes to actin cytoskeleton remodeling and regulation of actin filaments.
Rac1 is used by different types of microbial organisms to enter cells. This is also the case for the Mycobacterium bovis Bacille Calmette–Guerin (BCG) strain, which is used as a vaccine for tuberculosis. The BCG infection of primary airway epithelial cells has been shown to induce Rac1 up-regulation and to cause actin redistribution [57]. In bladder cancer cells, the entry of the BCG was found to rely on the expression of Rac1 and its effector kinase Pak1 (as well as Cdc42) via a process of micropinocytosis [58]. A BCG-induced enhanced expression of Rac1 has been reported in a study with infected macrophages, both in vitro and in vivo. The mycobacteria activated the p38K/JNK/b1-integrin/Rac1 signaling cascade in the frame of the infection [59].
The treatment of recurrent urinary tract infections usually relies on the use of intravenous antibiotic therapy (which can lead to complications due to allergy or drug-resistance). Repeated intravesical drug delivery is also possible, but it is more challenging [60]. The efficacy is these treatments is suboptimal at present. There is a need for new therapeutic options, new drugs and novel approaches in general to address the pathophysiology of the disease [61].
2.2. Rac1 and Diabetes-Induced Bladder Dysfunctions
Urinary bladder dysfunction is a complication in diabetes mellitus (DM) [62]. Diabetes causes bladder remodeling leading to uropathy in a mulitfactorial way, with neurogenic and myogenic detrusor overactivity and changes in transmitter regulation leading to a hyper-excitability of the detrusor [63]. DM is also a risk factor for bladder cancer prognosis and outcome [64]. Diabetic cystopathy (urinary disturbances) is one of the most common complications of diabetes mellitus [65]. The pathophysiology of the disease is complex and multifactorial, but it seems clear that Rac1 plays a role in the inflammatory mechanism, via binding to and the activation of the NOD-like receptor protein 3 (NLRP3) inflammasome. Indeed, under hyperglycemia conditions, Rac1 can promote NLRP3 inflammasome activation and induces cell damage [66]. The oxidative stress that occurs in the bladder of diabetic subjects causes oxidative damage to the urothelial and smooth muscle cells. A markedly enhanced expression of Pak1 (RAC1/p21 activated kinase 1) has been observed in the smooth muscle of diabetic mouse bladders versus the control group [67]. In a rat model of streptozotocin-induced diabetic bladder, an increased expression of Rac1 has been observed by immunohistochemistry. The Rac1 immunoreactivity was found to increase significantly in all the layers of the bladder tissue (epithelium, lamina propria, and tunica muscularis) for the diabetic group compared to the control group [68]. This study is important because it also showed that a Rac1 inhibitor (NSC23766) can inhibit the contractile responses of the bladder detrusor smooth muscle. This pharmacological aspect is discussed further below.
Interestingly, the expression of Rac1 in bladder tissue is increased not only due to the diabetes context, but it is also enhanced mechanically through the induced and cyclic hydrodynamic pressure exerted on bladder smooth muscle cells [69]. The expression of both Rac1 and phospho-Rac1 was found to be increased when a hydrodynamic pressure was mechanically applied onto human bladder smooth muscle cells. The expression of Rac1 downstream effectors, such as phospho-MEK1/2 and ERK-1/2, was also increased, and the effects were abrogated when cells were treated with a small molecule Rac1 inhibitor (NSC23766) or a Rac1 siRNA [69]. Rac1 seems to play an important role in the proliferation and response of bladder smooth muscle cells to hydrodynamic pressure. The data suggest that, in this situation, the use of Rac1 inhibitors could permit a reduction in bladder dysfunctions.
Another line of evidence showing that Rac1 plays an important role and is required for active contraction in smooth muscle comes from experiments using a conditional Rac1 knockout mouse strain. In this case, the loss of about 50% of Rac1 protein in the urinary bladder resulted in a significant decrease in the contractile responses to different agonists, without causing a remodeling of the vessels in the bladder tissue [70]. Similar effects were obtained using Rac1 inhibitors, as discussed below. In a recent study, the silencing of Rac1 expression in human bladder smooth muscle cells was found to reduce cell viability by 50–70% after 48 h and to increase the percentage of cells in (early/late) apoptosis compared to wild-type cells. The effects were associated with alterations in actin organization [71].
There are currently multiple pharmacological options to treat diabetes-induced bladder dysfunctions, notably using α1-adrenoceptor and muscarinic receptor antagonists, β3-adrenoceptor agonists and phosphodiesterase type 5 inhibitors [72]. However, here also, newer treatments and drugs are needed to improve long-term efficacy.
3. Rac1 in Bladder Cancer
The Rac1 gene, like other Rho-related genes, is frequently overexpressed in urothelial cell carcinoma, and the altered expression of the corresponding proteins plays an important role in the genesis and progression of cancers of the urinary bladder [73,74]. The gene overexpression and alterations not only concern Rac1 but also the associated regulatory elements, such as kinases PAK1 and PAK4 (P21 activated kinase 1/4), which are amplified and/or overexpressed in muscle-invasive bladder carcinomas [75,76,77]. A moderate or strong positive expression of both Rac1 and PAK1 are considered independent factors for shortened disease-specific survival time in patients with upper urinary tract urothelial carcinoma [78]. Numerous studies have reported alterations of Rac1 expression and function in bladder cancer, and the expressed protein has been associated with a variety of functional alterations. For the sake of clarity, we can refer to four categories of effects, briefly discussed in turn hereafter.
3.1. Rac1 in Bladder Tumorigenesis
A bioinformatic analysis of mRNA from patients with urothelial carcinoma of the bladder has revealed the presence of a shorter 3’-UTR (3′-untranslated region) isoform of Rac1 and this specific isoform was associated with an upregulation of Rac1 protein expression (Figure 4). The formation of this isoform was apparently mediated by the recruitment of the cleavage stimulation factor 2 (CSTF2) at a polyadenylation site of Rac1, thereby reducing the recruitment of two transcription factors (AFF1 and AFF4), thus causing defects in elongation. The short 3’UTR isoform of Rac1 apparently plays an essential oncogenic role in the pathogenesis of bladder cancer [79]. The enhanced expression of Rac1 is certainly not the sole key element contributing to bladder carcinogenesis; the modulation of the full Rho-GTPase axis has been implicated in bladder cancer tumorigenesis [80]. Rac1 plays a role in the carcinogenesis of various cancers, including bladder cancer but also hepatocarcinoma, breast cancer, non-small-cell-lung cancers and others [81,82].
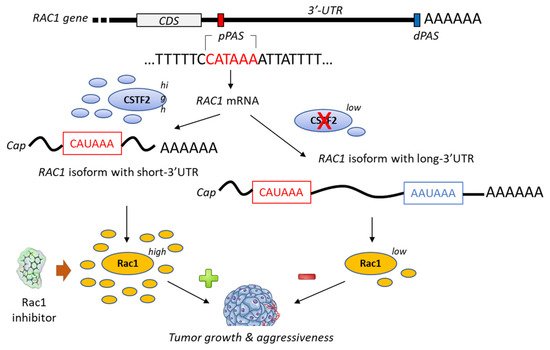
Figure 4. Proposed mechanism for the generation of short-3′-UTR (3′-untranslated region) isoform of Rac1 in the presence of a high level of the cleavage/polyadenylation factor CSTF2 (cleavage stimulation factor (2). The RAC1 short-3′UTR isoform has oncogenic functions and increases aggressiveness of cancer cells from urothelial bladder carcinoma UBC). The dual high expression of CSTF2 and Rac1 with short 3′-UTR predicts worse prognosis for UBC patients [79]. The proximal and distal polyadenylation sites (pPAS, dPAS) are located within the terminal exon. CDS, protein-coding sequence.
3.2. Rac1 in Bladder Cancer Cells Proliferation and Tumor Progression
The Rac1 protein is one of the elements that contributes to the proliferation and dissemination of bladder cancer cells. The Rac1 axis is a regulatory mechanism of bladder cancer progression. A recent study has shown that the adaptor protein RacGAP1 inactivated GTP-bound Rac1 in bladder cancer, but the activation was inhibited by protein SHCBP1 (SHC-binding protein 1), which is a regulator of EGF (epidermal growth factor). Via this relay, SHCBP1 can inactivate Rac1 and promote bladder cancer progression [83]. The EGF/EGFR ligand/receptor couple is frequently overexpressed in bladder cancers, with squamous bladder cancers qualified as being EGFR-addicted [84]. This trend encourages the use of EGFR-targeted drugs to treat these cancers [85,86]. EGFR signaling generally follows the PAK1/Rac1 route to convey the signal and to regulate tumor progression [87,88]. EGF is known to stimulate both Rac1 and Pak1 in vascular smooth muscle cells (Figure 5) [89]. It is therefore possible to slow down the proliferation and migration of bladder cancer cells via a brake on Rac1. This can be done directly with Rac1 inhibitors or indirectly with molecules capable of controlling Rac1 expression and function. This is the case, for example, for the microRNA miR-142-3p, which interacts directly with Rac1 in bladder cancer cells to inhibit their proliferation but also their migration and invasion [90].
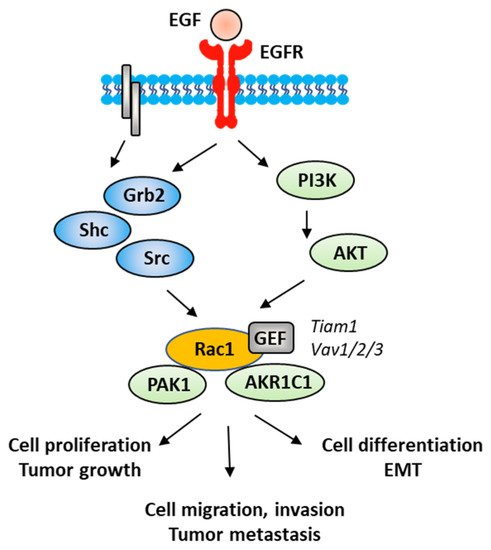
Figure 5. A signaling network between EGFR and Rac1, in vascular smooth muscle cells with various signaling effectors implicated.
3.3. Rac1 in Epithelial-Mesenchymal Transition (EMT) of Bladder Cancer Cells
EMT is a biological process through which epithelial cells lose their epithelial phenotype and gain mesenchymal features. This process reflects the aggressive and invasive character of the tumor and is often correlated with metastasis. EMT is vital for the progression of bladder cancer tumors because it plays a crucial role in cancer cells spreading and invasion [91]. Numerous signaling proteins contribute to this cellular differentiation process and Rac1 is one of them [92]. The targeting of the EGFR-Rac1 axis can permit to reverse EMT [93]. The activation of Rac1 in the frame of the EMT implicates various modulating proteins, such as SPAG9 (sperm-associated antigen 9) and HEF1 (human enhancer of filamentation 1), which are both connected to Rac1 expression [94]. Other factors are implicated in Rac1-mediated EMT, such as the metabolic enzyme AKR1C1 (aldo-keto reductase 1C1), which mediates the invasive potential and drug resistance of metastatic bladder cancer cells (Figure 5). The inhibition of AKR1C1 reduces the invasion/metastatic potential of bladder cancer cells via the regulation of the Rac1/Src/Akt pathway and modulation of the production of inflammatory cytokines, such as interleukin 1β (IL-1β) [95]. The link between EMT and Rac1 has been studied more deeply in other types of cancers, notably in lung and colon cancers [96,97]. The contribution of Rac1 to the inflammation process shall not be neglected. Different studies have pointed out a marked reduction in the production of inflammatory cytokines upon the inhibition of the activation of Rac1, directly or through intermediate effectors [98,99,100]. Rac1 is a major actor of the crosstalk between the inflammatory state and tumor cell migration [101]. The expression and activation of Rac1 has frequently been found to enhance the production of pro-inflammatory cytokines (IL-1β, but also IL-6, IL-8 and TNFα) in different pathological situations [102,103]. For example, the Rac1-GEF interaction inhibitor 1D-142 reduces the nuclear translocation of the transcription factor NFκB induced by the cytokine TNFα in NSCLC cells, and this activity contributes significantly to the antitumor effect of this guanidine-type Rac1 inhibitor in vivo [104]. Rac1 can interact directly with specific cytokines, such as IL-37, which controls the membrane translocation of the protein and its signaling activities, at least in lung adenocarcinoma [105]. In this context, more attention should be paid to the alternatively spliced isoform Rac1B, the expression of which can be induced by pro-inflammatory extracellular signals in polarized colorectal cancer cells [106,107]. Rac1 protects cells from undergoing EMT in pancreatic and breast epithelial cells. Similar studies should be conducted with bladder cancer cells.
3.4. Rac1 in Bladder Cancer Metastasis
The role of Rac1 activation in tumor metastasis has been amply discussed, notably in the frame of various solid tumor types [28,46,108]. A comparable situation can be underlined in bladder cancer. Rac1 is a major player of the metastasis of bladder cancer [109]. The invasion and migration of bladder cancer cells depend, to some extent, on the activation status of Rac1 and the activity of its regulators, notably the aforementioned Rac1-binding protein aldoketo reductase 1C1 (AKR1C1), up-regulated in metastatic human bladder cancer specimens. AKR1C1 antagonists, such as the anti-inflammatory drug flufenamic acid, can be used to decrease the invasion potential of metastatic bladder cell lines [95]. A high activity of GTP-bound Rac1 (coupled with high expression of Pak1) has been measured in the lymph node metastasis of urothelial carcinoma of the upper urinary tract, thus providing a potential prognostic marker for this disease, but also reinforcing the idea that targeting Rac1 can reduce dissemination of the tumor [110].
Rac1 plays roles in tumorigenesis, tumor progression, EMT and metastasis of bladder cancer cells. The GTPase has been also implicated in other hallmarks of cancer, notably in stemness, immune escape and drug resistance [30,111]. These aspects have not been significantly studied in the frame of bladder cancer. For these reasons, we will not discuss further these aspects here, but they provide additional indirect lines of evidence supporting the interest of targeting activated Rac1 in bladder cancer.
The management of bladder cancer is excessively complex and variable, depending on the tumor types and stages. Endoscopic transurethral resection of bladder tumor represents the standard of care for non-muscle invasive bladder cancer. However, for more advanced bladder cancers, chemotherapy is required. Immunotherapeutic strategies for bladder cancers have also been largely developed in recent years through the use of immune checkpoint inhibitors (antibodies), adoptive cell therapy, cytokine-based therapy and antibody–drug conjugates [112]. However, there is always a need for new drugs and combinations to improve treatment efficacy and patients’ survival.
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10061357
This entry is offline, you can click here to edit this entry!
