Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Immune checkpoint inhibitor therapy has shown a revolutionary therapeutic effect in various carcinomas. However, immune-related adverse events (irAE) after this treatment may lead to discontinuation of treatment. One such frequently encountered adverse event is immune-associated colitis (irAE colitis).
- immune checkpoint inhibitor
- immune-related adverse events
- irAE colitis
1. Corticosteroids
The first-line treatment for irAE colitis, as for most other irAEs, is CSs [59]. CSs inhibit the innate and adaptive immune systems by inducing apoptosis in activated T cells and inhibiting dendritic cell maturation [63,64]. In addition, CSs inhibit the production of pro-inflammatory cytokines from activated T cells, such as IL-2 and IFN-γ [65]. Furthermore, it has been demonstrated in mouse models that CSs enhance the surface expression of PD-1 in both CD4+ and CD8+ T cells and suppress their functions [66]. These findings can explain the effectiveness of systemic CSs for irAE colitis [67].
In general, CS treatment for irAEs is temporary, and CSs should be tapered off over 4 to 6 weeks when symptoms improve [59]. However, CSs do not necessarily lead to an immediate improvement in symptoms, which might also recur during tapering. Colon ulcers, entire colon inflammation, and a high Mayo score have been reported as predictors of patients with CS-refractory irAE colitis [29,67]. In addition, the molecular characteristics of aggressive irAE colitis are increased in the presence of group 3 innate lymphoid cells (ILC3s) in the mucosa and the intense infiltration of CD4+ and CD8+ T cells [45,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63, 64,65,66,67,68,69]. There are also reports suggesting that specific human leukocyte antigen (HLA) expression (HLA-B*35, DRB1*11) correlates with the risk of developing irAEs [70,71]. Furthermore, Coutzac et al. [68] showed a negative correlation between mucosal TNF-α expression levels and susceptibility to CS. Sakurai et al. [72] reported that the expression of genes involved in IFN-γ signaling are increased in the intestinal mucosa of patients with CS-resistant irAE colitis.
It should be noted that the application of long-term and high doses of CS increases the risk of complications such as osteoporosis, infections, and impaired glucose tolerance [73,74]. In addition, although CS is effective for irAEs, there is concern that the antitumor effect may be reduced by harmful mechanisms such as cytokine inhibition. Several reports indicate that the use of CS has no effect on patient survival [75,76,77]. Skribek et al. [77] examined the effect of CS on survival outcomes in patients with non-small cell lung cancer. In their cohort, the CS group (CS ≥ 10 mg, defined as ≥ 10 days) included 31 irAE patients, 10 of whom had irAE colitis with a severity grade of 2 or higher. They showed that CS administration to alleviate cancer-related symptoms was the only independent predictor of a reduction in survival, and that CS treatment for irAE had no effect on survival. Table 2 summarizes the papers reporting the association between the use of CS for irAE and cancer prognosis. Furthermore, Faje et al. [78] reported a reduction in survival in patients with malignant melanoma who received high-dose CS treatment (CS ≥ 7.5 mg, defined as ≥2 months) for irAE pituitary. Therefore, whether CS affects the survival of patients with irAE remains controversial. CS exposure should be minimized, taking into account various complication risks and unpredictable prognostic effects. For irAE colitis in CS-resistant cases, alternative courses of treatment should be considered.
Table 2. Association between CS use for irAEs and cancer prognosis.
| author | year | Pathogenic diseases | No. Case (CS Naïve: Need CS) |
Effects of CS on response rate or survival |
|---|---|---|---|---|
| Horvat et al. [75]] | 2015 | melanoma | 195:103 | Systemic CS was not associated with OS or TTF |
| Weber et al. [76]] | 2017 | melanoma | 462:114 | The ORR was 31.8% in the CS naïve group and 29.8% in the CS required group (p = 0.736). The median duration of response was 22.0 months in the CS naïve group and was not reached in the CS required group. |
| Skribek et al. [77]] | 2020 | lung cancer | 104:31 | OS was 14.43 months in CS naïve group and not reached in CS required group (p = 0.38) |
CS, corticosteroids; OS, overall survival; TTF, time to treatment failure; ORR, overall response rate.
2. Infliximab
ASCO Guidelines, NCCN Guidelines, and the Cancer Immunotherapy Society (SITC) Toxicity Control Working Group recommend IFX for CS resistant cases [58,59,79 ]。 IFX is an anti-TNF α monoclonal antibody that has been reported to be very effective in IBD (ie, Crohn's disease and UC). Several case reports and retrospective studies have also shown its effectiveness in irAE colitis [56,60,61,67,80,81,82,83,84]. TNF-α signaling is heavily involved in cellular functions such as cell migration, proliferation, and apoptosis.
The impact of IFX on the anti-tumor effect of ICI is controversial. Badran et al. showed that five patients with CS-resistant irAE colitis could achieve both disease control and colitis control with a combination of IFX and ICI [94]. Lesage et al. [82] and Wang et al. [15] also reported that the use of IFX for irAE colitis had no effect on survival. In contrast, Verheijden et al. [95] compared the survival rates of the CS-only group and the IFX-treated group in all irAE-affected patients studied, and showed that overall survival was reduced in the IFX-treated group. Chen et al. [96] reported that TNF α inhibitors enhance the antitumor activity of ICI by promoting cytotoxic T cell (CTL) activity and may exert a direct cancer-inhibiting effect by inhibiting regulatory T cell (Treg) function. However, TNF-α inhibition has a direct effect on tumorigenesis, while long-term use of TNF-α inhibitors may prevent the differentiation of naïve CD8+ T cells into CTL and deplete antitumor CTL cells. Although there are no reports that the administration of IFX for irAE colitis directly exacerbates the underlying disease, long-term use of IFX should be avoided and IFX administration should be discontinued once remission is achieved.
3. Bedridumab
VED is an IgG1 monoclonal antibody that specifically binds to α4β7 integrin on activated T cells. It inhibits the entry of activated T cells into intestinal tissue by blocking the interaction with mucosal addressing-in cell adhesion molecule-1 (MAdCAM-1), which is selectively expressed in intestinal vascular endothelial cells [97, 98]. The efficacy of VED has been demonstrated in IBD [99]. The dataset available for VED is smaller than IFX, but ASCO and NCCN guidelines present it as a treatment option next to IFX [58,59].
There are no reports of direct comparisons of clinical trials of IFX and VED treatment for CS refractory irAE colitis.
4. Other Therapeutics
The therapeutic effect of mofetil mycophenolate (MMF) [30,105], calcineurin inhibitors (tacrolimus [106,107] and cyclosporine [108]), and tocilizumab [109] has also been reported.
MMF exerts an immunosuppressive effect by inhibiting inosine-5'-monophosphate dehydrogenase (IMPDH) and inhibiting the replication of T and B cells. Mir et al. [105] reported 11 cases of irAE colitis treated with MMF in combination with CS. The remaining four patients who relapsed responded strongly to IFX.
Calcineurin inhibitors (CNI) (tacrolimus and cyclosporine) bind to calcineurin by forming an intracellular complex with FK506-binding protein 12, inhibit the release of cytokines such as IL-2, TNF-α, and IFN-γ, and exhibit a robust immunosuppressive effect by inhibiting T cell activation. Calcineurin inhibitors are commonly used in patients with moderate to severe UC [110]. The British Society of Gastroenterology (BSG) and the European Society of Oncology (ESMO) recommend the use of tacrolimus for irAE colitis [111]. Kunogi et al. [106] reported cases of improved diarrhea after tacrolimus administration for irAE colitis refractory to CSs, IFX, and VED. In their report, tacrolimus was effective for irAE, but liver metastases appeared 3 months after tacrolimus administration.
Tocilizumab, an anti-IL-6 receptor antibody, is an established treatment for moderate to severe rheumatoid arthritis (RA). IL-6 promotes inflammation via trans signaling pathways,[112] and is known to promote tumor progression and metastasis through a variety of mechanisms, including activation of tumorigenic pathways and inhibition of dendritic cell differentiation. ]。 Thus, IL-6 inhibition may be compatible with tumor suppression and cancer-related symptom management. Stroud et al. reported 34 cases of CS refractory irAE in patients treated with tocilizumab. Of these, only one patient suffered from irAE colitis, and tocilizumab relieved symptoms without affecting survival. When using tocilizumab, it should be noted that an increased risk of intestinal perforation has been reported in clinical trials in patients with RA. In particular, patients with ulcerative lesions of the stomach or intestine who continued long-term CS treatment had an increased risk of intestinal perforation [114]. Therefore, in patients with a history of long-term CS administration or severe gastrointestinal ulcers with irAE colitis, tocilizumab should be administered with caution.
5-Aminosalicylic acid (5-ASA) is a commonly used drug for IBD that acts topically on the colonic epithelium. The anti-inflammatory effect of 5-ASA is exerted mainly by inhibiting cyclooxygenase and lipoxygenase, followed by a decrease in the production of prostaglandins and leukotrienes [115]. The nuclear receptor peroxisome growth factor activated receptor lig γ and (PPAR-α), a transcription factor that inhibits TNF-γ production, is activated by 5-ASA [116]. There have been reports of administration of 5-ASA to existing UC patients with irAE [117], but its efficacy is unknown.
FIG. 1 shows the mechanism of action of CS and biologics against irAE colitis.
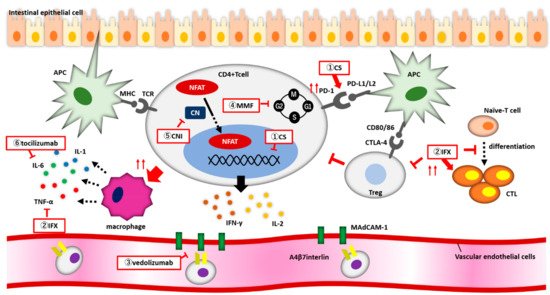
図1.irAE大腸炎に対するCSおよび生物製剤の作用機序。(1)コルチコステロイド(CS)は、CD4+T細胞の表面におけるPD-1発現を増強し、CD4+T細胞の核内受容体に結合し、炎症性サイトカインの放出を抑制する。(2)インフリキシマブはCTL活性を増強し、Treg機能を抑制し、ナイーブT細胞がCTLに分化するのを阻害する(3)ベドリズマブはα4β7インテグリンのMadCAM-1への結合を阻害し、CD4+ T細胞が血管から腸内に移動するのを阻止する。(4)MMFはIMPDHを可逆的かつ特異的に阻害し、かつリンパ球が細胞周期のG1~S期の間に増殖を阻止する。(5)カルシニューリン阻害剤は、NFATが核に移行するのをブロックし、炎症性サイトカイン遺伝子の発現を低下させる。
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10061334
This entry is offline, you can click here to edit this entry!
