Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Benzo[a]pyrene (B[a]P) is the main representative of polycyclic aromatic hydrocarbons (PAHs), and has been repeatedly found in the air, surface water, soil, and sediments.
- benzo[a]pyrene
- polycyclic aromatic hydrocarbons
- metabolism
1. General Information
Benzo[a]pyrene (B[a]P) is one of the representatives of aromatic hydrocarbons. Having five benzene rings, B[a]P belongs to a group of polycyclic aromatic hydrocarbons (PAHs). Depending on the place of the fifth benzene ring attachment, we can distinguish B[a]P (a single benzene molecule attached at the bond a) or benzo[e]pyrene (a single benzene molecule attached at the bond e) (Figure 1). A single molecule of benzene is liquid, while benzopyrene is solid. PAHs containing two or more fused benzene rings form stable molecule structures of a high hydrophobic nature [1].
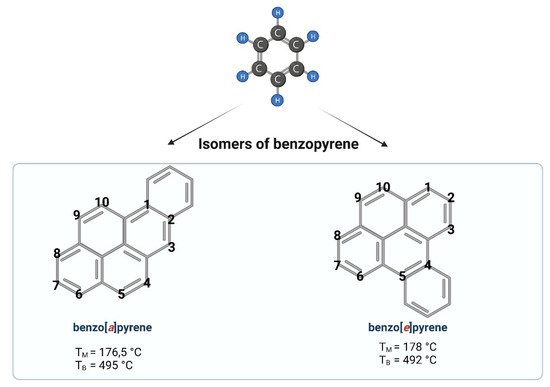
Figure 1. Chemical structures of benzopyrene. Created with BioRender.com (accessed on 12 April 2022).
B[a]P and other PAHs have been shown to be formed during the burning of fossil fuels, wood, and other organic materials. B[a]P has been detected in high levels in cigarette smoke, diesel exhaust, charcoal-based foods, as well as industrial wastes [2]. It is therefore a substance that is formed as a by-product in various thermal processes.
The exposure to B[a]P in recent times is more common than ever before. Sources of B[a]P emissions are mostly anthropogenic, and to a lower extent natural, including wildfires and volcanic eruptions [3].
In mammals, B[a]P is readily absorbed after inhalation, oral administration, and through the skin [4]. The main sources of human B[a]P exposure are contaminated food and the air [5].
An important source of exposure to B[a]P is tobacco smoke. B[a]P concentrations in the side stream of cigarette smoke was shown to be in the range of 52 to 95 ng/cigarette—over three times higher than in mainstream smoke [6]. High concentrations of B[a]P, and particularly its metabolite—7β,8α-dihydroxy-9α,10α-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene (BPDE)—have been determined in smokers. Susanto et al. [7] detected a higher level of BPDE protein adducts in smokers (8.87–33.55 ng/mL) compared to nonsmokers (3.87–13.27 ng/mL). BPDE DNA adducts were also determined in the placenta of smoking mothers [8]. In smokers (compared to nonsmokers), the exposure to B[a]P present in tobacco smoke leads to a prolonged gestation period, earlier mean menopausal age, altered ovarian steroidogenesis, and ovarian reserve depletion [9].
2. Occupational Exposure to PAHs, Including B[a]P
B[a]P is usually present in the highest concentrations in the mixture of PAHs (emitted during combustion and other technological processes) to which workers in many branches of industry are exposed. Therefore, when evaluating the correlation between the incidence of a given disease (including people representing specific occupational groups) and the exposure to PAHs mixtures, it is justified to relate this association indirectly to B[a]P.
Occupational exposure to PAHs, including B[a]P, occurs mainly through inhalation and dermal contact. The highest mean level of PAHs has been determined in aluminum production (Söderberg process) with concentrations up to 100 μg/m3. Average levels of PAHs are detected in roof coverings and pavements (e.g., 10–20 μg/m3), and the lowest (i.e., 1 μg/m3 or below) have been noted in coal liquefaction, coal-tar distillation, wood impregnation, chimneys, and power plants [6].
Saleh et al. [10] assessed differences in the PAHs, including B[a]P concentrations, in samples of air inhaled by occupationally exposed and nonexposed groups inhabited in Makkah (Saudi Arabia). They detected B[a]P in the mean concentration of 0.082 ± 0.032 ng/m3 in occupationally exposed workers (bus and truck drivers, police officers, etc.), while its mean concentration in unexposed group was 0.044 ± 0.006 (Table 1). Interestingly, they observed a positive correlation between increased B[a]P exposure and serum p51 and p21 proteins levels that are considered to be implicated in tumor progression, invasion, and metastasis.
Table 1. B[a]P levels in outdoor air.
| Location | Concentration [ng/m3] | Reference |
|---|---|---|
| The European Union | 7% of EU citizens live in areas with a tolerable risk level of 0.12 ng/m3 | [12] |
| France | 1 ng to 2.49 ng/m3 | [13] |
| Thailand | 0.052 and 0.095 ng/m3 in PM 2.5 fraction | [14] |
| Iberian Peninsula | exceeded target value of 1 ng/m3 | [15] |
| Italy—Genoa | 2 ng/m3 (along heavy traffic streets) | [16] |
| 14 ng/m3 (300 m from a coke oven) | ||
| Poland—Cracow | 4–10 ng/m3, | [17] |
| Tarnow | 4–6 ng/m3 | |
| Nowy Sacz | 10–11 ng/m3 | |
| China—Linzhou | 5.1–20.2 ng/m3 | [18] |
| Saudi Arabia—Makkah | 0.082 ± 0.032 ng/m3 (occupationally exposed workers) | [10] |
| 0.044 ± 0.006 ng/m3 (unexposed group) |
In another work, Petit et al. [11] have recently evaluated the incidence of lung cancer risk related with PAHs exposure in various industries using atmospheric B[a]P level as a surrogate value. They analyzed a total of 93 exposure groups, which belonged to nine branches of industry. They have shown that 30% of exposure groups were above the maximum risk level of the European Union (10−4).
2. Metabolism of B[a]P
B[a]P metabolism in humans was studied by [73]. The researchers gave B[a]P a microdose orally to humans and observed its rapid uptake (Tmax, 0.5–1 h), complex metabolism (predominantly at the B[a]P bay region), and fast removal (mean half-life—46.5 ± 58.2 h) from the body. Parent [14C]-B[a]P was a minor component in plasma as compared to the sum of metabolites, and decreased along with the dose. Pharmacokinetic parameters for almost all metabolites showed a linear dose–response correlation. They also observed interindividual differences in pharmacokinetics within doses of 25–250 ng.
The metabolism of B[a]P involves several phases, as it also is in the case of many other hydrophobic xenobiotics (Figure 2).
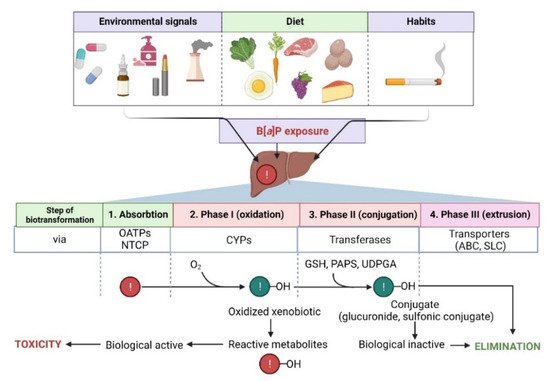
Figure 2. Steps in the biotransformation of B[a]P. Detoxification of B[a]P predominantly occurs with the participation of several cytochrome P450 (CYPs) isoforms. During phase I, xenobiotic is oxidized to reactive metabolites that may exhibit biological activity. In phase II, metabolites are conjugated with transferases (GSH, reduced glutathione; PAPS, 3′-phosphoadenosine 5′-phosphosulfate; UDPGA, uridine diphosphate-glucuronic acid) and converted to more hydrophilic and biologically inactive forms. Then, metabolites are eliminated from the cell via specialized transporters (ABC, ATP-binding cassette transporter; SLC, solute carrier). NTCP, sodium/taurocholate cotransporting polypeptide; OATPs, organic anion transporting polypeptides—biological systems enabling the transport of xenobiotics to the cell [74,75]. Created with BioRender.com (accessed on 12 April 2022).
The first phase of biotransformation of PAHs, including B[a]P, involves the activity of cytochrome P450 (CYP1 family) and of microsomal epoxide hydrolase. During the first phase, PAHs are transformed to some phenols (hydroxy derivatives), phenol diols, dihydrodiols, quinones, and reactive-diol-epoxides enantiomers. During PAHs transformation, ROS are also produced as by-products of these reactions.
Several enzymes acting within cytochrome P450, including CYP1A1, CYP1A2, and CYP1B1, have been shown to be implicated in B[a]P oxidation, while CYP1A1 is considered to be the most active in mammals [76,77]. B[a]P epoxidation by P450 at the 7,8 positions has been found to be one of the most dangerous reactions, which leads to the formation of B[a]P toxic metabolites. Interestingly, B[a]P oxidation at the 4 and 5 positions leads to the creation of an inactive metabolite, which is eliminated from the organism [78]. As mentioned above, CYP1A1 can convert B[a]P to B[a]P-7,8-epoxide, which (in the presence of epoxide hydrolase) is transformed to (+/−)-B[a]P-trans-7,8-dihydrodiol (DHD). B[a]P-7,8-DHD is a substrate for the reaction of the second CYP-dependent oxidation, which forms the final carcinogenic metabolite—7β,8α-dihydroxy-9α,10α-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene (BPDE). It has been shown that in the cell nucleus, diol-epoxides can bind covalently to DNA, creating deoxyguanosine-DNA adducts that may lead to erroneous replication and mutagenesis [76,78] (Figure 2). Metabolism of B[a]P may therefore lead to the production of electrophilic metabolites exhibiting a carcinogenic potential. Cytochrome P4501A1 (CYP1A1) is believed to play the most important role in the pro-carcinogenic B[a]P activation necessary for the formation of DNA adducts. Shiizaki et al. [79], in a review paper, described factors that could affect the formation of B[a]P-DNA adducts and hypothesized that CYP1A1 was a key enzyme in the production of (BPDE), the major carcinogenic intermediate of B[a]P (Figure 3).
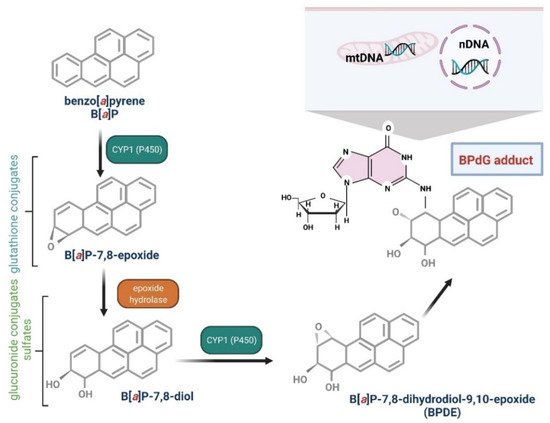
Figure 3. Biotransformation of B[a]P into mutagen. B[a]P in the I phase of detoxification goes through several stages, giving various derivatives, including B[a]P-7,8-diol-9,10-epoxide, that are able to react with DNA guanine. The reaction results in the formation of adducts with DNA (mtDNA, mitochondrial DNA; nDNA, nuclear DNA) and mutations [80,81]. Created with BioRender.com.
AhR activators, such as 2,3,7,8-tetrachloro-dibenzo(p)dioxin, may potentially increase B[a]P toxicity through induction of the CYP1A1 gene. At the same time, it is believed that inhibitors of CYP1A1, including its substrates, may reduce the toxicity of B[a]P [76,79].
Phase I metabolism of B[a]P may not only be mediated by CYP but also by flavin-containing mono-oxygenases, NAD(P)H: quinone oxidoreductases, amine oxidases, esterases, alcohol dehydrogenases, and peroxidases [76]. Prostaglandin H synthase and lipoxygenase also participate in metabolic transformations of B[a]P, being responsible for the formation of highly reactive, oxygen-free radicals [82]. In the presence of polyunsaturated fatty acids, including linolenic and arachidonic acid, B[a]P undergoes oxidative transformations, and the resulting oxidized B[a]P and lipid peroxidation products demonstrate mutagenic potential. Thus, products of B[a]P oxidation and polyunsaturated fatty acid peroxidation may participate in mutagenesis and may also induce carcinogenesis [83].
In the second phase, products of the first phase are conjugated with endogenous compounds, e.g., GSH, glucuronic, and/or sulfate acids, creating GSH conjugates, glucuronides, and sulfate esters that are, due to their hydrophilic nature, more efficiently eliminated from the body [84] (Figure 2).
In the third phase, an important role is played by ABC proteins, such as ATP binding cassette (e.g., multidrug resistance protein family—MRP) [85] (Figure 2).
This entry is adapted from the peer-reviewed paper 10.3390/ijms23116348
This entry is offline, you can click here to edit this entry!
