Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The management of the microbial infection bioburden and tissue remodelling is a crucial aspect of wound care. Compounds with wound healing properties are effective under certain conditions, including low concentrations; however, a lethal concentration has been reported to have safety concerns, ranging from prooxidant effects to DNA damage.
- antimicrobial
- anti-inflammatory
- antibiotics-free
- wound care
- tissue remodelling
1. Curcumin
Curcumin (Figure 1) is a lipophilic, bioactive compound obtained from the rhizome of the Curcuma longa Linnean plant, [1]. It is a phenolic dye with a bright yellow colouration constituting the major component of the curcuminoid of turmeric (Curcuma longa), accounting for the yellow colouration observed in turmeric [1]. Traditionally, it is used as an adjuvant (E100) in the food industry as a colouring and flavouring agent [1]. Beyond its traditional applications, the polyphenolic component of curcumin is known to actively regulate several signalling pathways and elicit a broad range of pharmacological activities [2], including anti-inflammatory [3][4][5], antioxidant [3][6], anticancer [7][8], antidiabetic [9], antiviral [10][11], and antibacterial activities [12][13][14][15][16][17][18]. Additionally, curcumin has been explored in the management of skin diseases, including psoriasis [19][20].
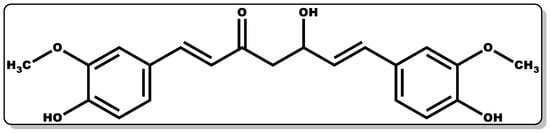
Figure 1. Molecular structure of (1E,6E)–1,7–Bis(4–hydroxy–3–methoxyphenyl)hepta–1,6–diene–3,5–dione (Curcumin).
Curcumin possesses sparing to low aqueous solubility and poor stability, and this has limited its broad applicability when administered alone [21]. Moreover, the stability of curcumin is pH-dependent, with pH 7 to 8 accounting for about 90% of its degradation and a slightly acidic pH of 3 to 6.5 affording better stability in comparison to pH 7 to 8 [22]. Interestingly, some of its metabolites have been demonstrated to possess fascinating pharmacological dispositions, including anti-inflammatory, antimicrobial, anticancer, and cardioprotective properties [3][7][23]. The incorporation of curcumin with nanomaterials, micelles, and their micronised forms has been reported to exhibit improved solubility compared to it pristine form [24][25].
In vitro data have demonstrated the antibacterial and wound healing potential of curcumin [17][18][26]. In vitro study data of curcumin against Escherichia coli and Bacillus subtilis FtsZ demonstrated significant efficacy [17][18]. Comotto and co-workers explored curcumin in combination with t–resveratrol in the fabrication of an alginate-based breathable hydrogel dressing for the treatment of infected wounds; this combination was found to exhibit pivotal bactericidal activity [26]. The rising preclinical data on curcumin’s medicinal disposition have endeared the interest of researchers both in academia and industry to the exploration of its potential clinical administration in the management of diverse disease conditions [4][13][23][27]. The potential of curcumin in wound care was evaluated using various models, including rats, and curcumin was shown to be instrumental in improving epithelialisation, fibroblast proliferation, vascular density, collagen deposition, and reorganisation [28][29][30][31][32]. This was demonstrated in a study by Mehrabani et al. where curcumin was shown to foster wound healing by quenching free radicals and the subsequent modulation of inflammation through the inhibition of nuclear factor-B. Furthermore, it accelerates the regulation of collagen deposition and fibroblast migration by inducing transforming growth factor-β and stimulating angiogenesis and extracellular matrix accumulation, which are essential for tissue regeneration [28]. In another study by Miah et al., curcumin was applied to surgical wounds of Bengal goats, and it showed better wound recovery compared to the untreated groups [31]. The combination of curcumin in formulation with other molecules has proven to be advantageous in improving its solubility and efficacy. A recent study by Schiborr and co-workers demonstrated the improved solubility of curcumin when it was incorporated with polysorbate [25]. Moreover, enhanced antibacterial and wound care efficacy of curcumin has been reported when combined with hyaluronic or t-resveratrol [26][30][33]. This was shown in a study conducted by Sharma et al., where curcumin combined with hyaluronic acid was tested against bacteria and diabetic mice, and it exhibited bactericidal activity and rapid wound healing efficacy when compared with the untreated groups [30].
The anti-inflammatory mechanism of action (MOA) of curcumin could be due to its regulation of the gene expression of inflammatory cytokines capable of releasing high influxes of tumour necrosis factor (TNF), interleukin-6 (IL-6), and nitric oxide (NO), which could cause persistent inflammation [23]. Moreover, its non-specific antimicrobial MOA against bacteria likely works by binding to the FtsZ proteins, leading to the inhibition of the FtsZ protofilaments assembly, thereby suppressing bacterial growth and proliferation. In addition, its mode of action may be attributed to its disruption of the mecA gene transcription, resulting in a decrease in penicillin-binding protein-2α expression. For instance, this is demonstrated when curcumin binds with the peptidoglycan on the S. aureus cell wall, making it unavailable for the production of the new peptidoglycan, affecting the strength of the peptidoglycan layer, and triggering the breakdown of the bacterium [13][27]. The MIC (values in bracket, Table 1) of curcumin against Staphylococcus aureus, Porphyromonas gingivalis, Escherichia coli, Staphylococcus epidermidis, Pseudomonas aeruginosa, Streptococcus mutans, Proteus mirabilis, Serratia marcescens, and Bacillus subtilis [27] is summarised in Table 1.
Table 1. Minimum inhibitory concentration (MIC) of curcumin and PHMB on various species of bacteria [27][34].
| Curcumin (MIC Values in %) | |||||||||
| S. aureus | P. gingivalis | E. coli | S. epidermidis | P. aeruginosa | S. mutans | P. mirabilis | S. marcescens | B. subtilis | |
| 0.0188 | 0.0125 | 0.0192 | 0.0175 | 0.0192 | 0.0175 | 0.0192 | 0.0384 | 0.0100 | |
| PHMB (MIC Values in %) | |||||||||
| S. aureus | P. gingivalis | E. coli | S. epidermidis | P. aeruginosa | M. luteus | M. smegmatis | S. enterica typh | B. subtilis | S. griseus |
| 0.0002 | 0.0010 | 0.0002 | 0.0001 | 0.0010 | 0.0010 | 0.0012 | 0.0004 | 0.0005 | 0.0005 |
2. Poly(hexamethylene biguanide)
PHMB (polyhexanide, Figure 2) is a known antiseptic with proven activity in the management of microbial infections. An increase in the polymer chain length of PHMB often leads to an improvement in its antimicrobial activity, and this structural chain length can be repeated 2 to 30 times [35][36]. PHMB has demonstrated high efficacy over a broad spectrum of microbes, including certain viruses [37][38], Gram-positive and Gram-negative bacteria [39][40], fungi [39][41], and certain parasites, particularly Acanthamoeba [42][43]. Its application has evolved beyond its traditional use as a multi-purpose disinfectant and deodoriser. Recent application of PHMB has been demonstrated in cosmetics and personal hygiene products as preservatives, with a concentration limited to 0.1% [44]. It is a synthetic polymer composed of a biguanide and hexamethylene moieties with structural similarity to naturally occurring antimicrobial peptides, giving it ease of penetrating bacterial cell membranes and eliciting bactericidal activity [45].
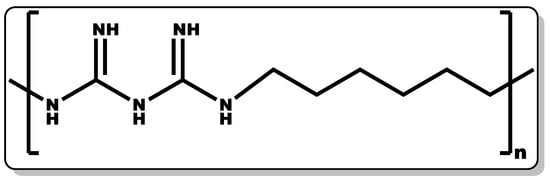
Figure 2. Structure of poly(hexamethylene biguanide).
Specifically, it is known to mainly target the outer and cytoplasmic membranes. PHMB binds to the DNA and other nucleic acids of the cell membrane, leading to the destruction or inactivation of the bacterial DNA [35][46]. There is growing evidence of its wound healing efficacy when alone and incorporated in wound care products, including cleansing solutions, hydrogels, and dressings [35]. Preclinical data have suggested that PHMB possesses efficacy against wound–colonising bacteria, including MRSA and other pathogenic bacteria [47]. The PHMB MIC values against various pathogenic bacteria are listed in Table 1 [34].
Numerous studies have shown PHMB’s therapeutic activity in wound care management [48][49]. Wound-care products containing PHMB were found to exhibit anti-inflammatory dispositions by decreasing wound pain and malodour [48][49][50]. Moreover, it increases keratinocyte and fibroblast activity with improvement in granulation tissue formation and the elimination of dead tissues in the wound [48][51][52]. In a study by Lenselink and co-workers, 28 volunteers with critically colonised wounds were recruited and placed on PHMB-containing formulations. An increase in tissue granulation was observed within 24 weeks, and this was attributed to the antioxidant, anti-inflammatory, and antibacterial efficacy of PHMB [53]. In another study by Elzinga and co-workers, the tolerability and healing efficacy of PHMB were evaluated, and it was demonstrated to be well-tolerated and afford pain-free wounds with a good recovery timeframe [54]. However, PHMB has been reported to have detrimental effects at high concentrations, including fever and a generalised exanthema, which is thought to be the promotion of high nitric oxide by PHMB [55]. According to the ECHA, 0.1% PHMB is considered safe for application in cosmetics formulations [44].
3. Vitamin A
Retinoids are a group of compounds with a lipophilic non-aromatic β-ionone ring having an unsaturated isoprenoid side chain (Figure 3). This class of chemical compounds consists of retinol and its derivatives, which are known for their pharmacological and physiological roles. These include the treatment of vision impairment and skin disorders, such as photodamage, acne vulgaris, wrinkles, and psoriasis [56][57][58][59]. Furthermore, they have demonstrated efficacy in the management of other skin abnormalities, such as disordered fibrotic proliferation, including hypertrophic scars, keloids, and scleroderma [59][60]. They continue to play an essential role in efficient epithelial keratinisation through the regulation of the proliferation and differentiation of several cell types within the skin, including keratinocytes and fibroblasts [59][61]. Additionally, they have been shown to modulate gene transcription by controlling the extracellular matrix (ECM) through elevated collagen and fibronectin generation coupled with decreased collagenase activity and the recruitment of local inflammatory mechanisms to foster wound healing [59]. Moreover, retinoid offers protection against ultraviolet–B (UVB)-induced DNA damage [62][63][64][65]. Retinoids’ crucial functionality in the epithelialisation and subsequent wound healing of compromised skin tissues is well documented [59]. Retinoids can be classified into four generations [58], which are listed as follows: (i) retinol, retinaldehyde (retinal), retinoic acid (tretinoin), isotretinoin, and alitretinoin belong to the first-generation class of retinoids, (ii) etretinate and its metabolite acitretin are the second generation, and (iii) the third-generation class includes bexarotene, tazarotene, and adapalene. Finally, (iv) the fourth generation includes trifarotene. However, the focus of these content will be on the first-generation retinoids, with particular emphasis on retinol, retinal, and retinoic acid (Figure 3).
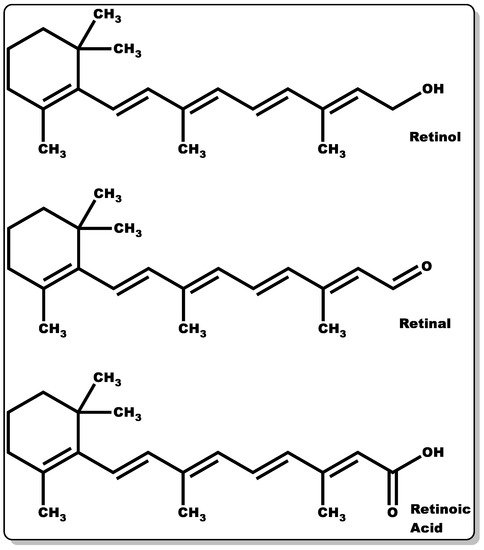
Figure 3. Molecular structures of retinol, retinaldehyde, and retinoic acid.
According to Törmä and co-workers, 90% of the retinoids in the skin are made up of retinyl ester, and retinol accounts for only 10% [66]. They have been reported to have a high capacity to absorb ultraviolet beam (UVB) radiation ranging from 300 to 350 nm [66]. This was demonstrated in a study by Antille and co-workers, where they investigated the skin photo-protection capacity of retinyl palmitate in the presence of high UVB radiation exposure. The outcome of the findings corroborated the photo-protection of the epidermis and anti-photocarcinogenic properties of retinyl ester [67].
Moreover, in a study by Pechère and co-workers where retinol and its natural derivates (retinal and retinoic acid) were tested against bacterial strains, only retinal and retinoic acid demonstrated inhibitory activity against S. aureus or P. acnes, with retinal affording more potent antibacterial activity compared to retinoic acid. The MIC of retinal against various Gram-positive bacteria (strains) is presented in Table 2 [68][69].
Table 2. Minimum inhibitory concentration (MIC) of retinoids against various strains of Gram-positive bacteria [68][69]. Methicillin-sensitive S. aureus (MSSA) and methicillin-resistant S. aureus (MSSA). Not active (NA).
| P. acne Strains | S. aureus Strains | ||||
|---|---|---|---|---|---|
| CIP179 | CIP53119 | CIP53117 | MSSA | MRSA | |
| Retinal | 0.0004% | 0.0004% | 0.0008% | 0.0008% | 0.0004% |
| Retinoic acid | 0.0128% | NA | |||
However, a recent study by Harris et al. showed that retinol can serve as a good agent in the prevention of microbial infections, particularly against S. pyogenes. [70]. The antimicrobial mechanism of action of retinal is thought to be the interaction of the adamantane component of retinal with the lipophilic layer of the bacterial cell membrane, thereby causing the disruption of its biosynthetic pathway [58]. Retinoic acid has been demonstrated to inhibit inflammatory reactions at the homeostasis phase by regulating the gene expression of inflammatory infiltrates and proinflammatory cytokines (tumour necrosis factor (TNF), interleukin-6 (IL-6), and nitric oxide (NO)) that could cause persistent inflammation [71]. Essentially, it can be postulated that there is a synergy in the pharmacological and physiological actions of retinol and its metabolites for wound healing; the retinal component has been proven to inhibit bacterial growth and proliferation, with retinoic acid modulating the homeostasis phase by regulating the influx of inflammatory infiltrates responsible for persistent inflammation and retinol modulating the growth factor expression essential for tissue regeneration [59][68][69][70][71].
3.1. Retinol
Retinol was first isolated from Scombresox saurus liver oil by Karrer in 1931 [72] (Figure 3). The compound is naturally found in animal products, and as chemical precursors in fruits and vegetables. Retinol is a fat-soluble molecule with antioxidant and wound-healing dispositions. Retinol, in comparison to its metabolites, does not have the same profound pharmacological and physiological properties [73]. Excessive administration of retinol could lead to skin irritation, such as erythema, dryness, peeling, pruritis, and stinging/burning [74]. For retinol to exert similar pharmacological and physiological responses comparable to those of its metabolites, a higher dose of retinol may be required, which could lead to adverse effects [75]. These adverse effects could be due to the excessive stimulation of epidermal turnover and cell proliferation, leading to hyperplasia and spongiosis (localised swelling of the epidermis) [76]. The ideal concentration of retinol can cause an increase in the epidermal thickness [77], which can occur through several processes. This could occur by the upregulation of genes related to collagen type I (COL1A1) and III (COL3A1), which in turn increase the protein expression of procollagen I and III [75]. The improved collagen production can reduce fine wrinkles and scar formation [65]. Using a 1% topical retinol application, increased fibroblast growth, increased collagen synthesis, and a reduction in matrix metalloproteinases were observed, all of which counteract the effects of photoageing or natural ageing in the skin [62]. As well as having a direct effect, retinol can increase the expression of cellular retinoic acid-binding protein II (CRABPII) [77], cellular retinol-binding protein (CRBP) mRNA, and protein [75]. The skin thickening disposition of retinol was demonstrated in a study by Kang and co-workers, where they applied all-trans-retinol to the healthy human epidermis, and it fostered epidermal thickening and elevated the mRNA expression of cellular retinoic acid and retinol-binding protein [76]. In another study by Varani and co-workers, the topical administration of retinol was found to decrease matrixins expression and elevate fibroblast proliferation and the production of collagen in naturally aged skin, as performed in photoaged skin [62][73]. Matrixins, also known as matrix metalloproteinases, are enzymes capable of degrading ECM and they play vital roles in cell growth, migration, differentiation, angiogenesis, apoptosis/necrosis, and host defence [78]. Essentially, they have been reported to influence the physiological or pathological functioning of the biological system, including metastasis, inflammation, and wound healing (tissue remodelling—angiogenesis, and epithelialisation) [78][79].
Retinol deficiency can lead to a general impairment of wound healing, characterised by delayed epithelialisation [59], and can lead to abnormal epithelial keratinisation [61]. This has been proven in a rat model [80]. Steroids are known to contribute to wound healing delay. In a study by Ehrlich and co-workers, the inhibitory activity of retinol against anti-inflammatory steroids was demonstrated, which could play a profound role in the wound recovery timeframe [81].
3.2. Retinal
Retinal was first isolated in 1934 by Wald and, in 1944, Morton suggested that the compound in question was vitamin A aldehyde, linking it to the previously recognised retinol [82] (Figure 3). Retinal is obtained by the hydrolysis of β-carotene, a retinoid precursor that is found in many fruits and vegetables.
The antibacterial activity of retinal was demonstrated by Pechère et al. where 0.05% of retinal was topically administered against Propionibacterium acnes and significant bactericidal activity was observed [68]. Retinal showed significant in vitro antibacterial activity against Gram-positive bacteria; however, there was no observed activity found against Gram-negative bacteria. It is hypothesised that the antibacterial effect is, in part, due to the aldehyde group in the lateral chain [68].
Much of the other effects of retinal are indirect, such that the functionality is from nuclear receptor binding, hence leading to gene modulation [69].
3.3. Retinoic Acid
Retinoic acid (Figure 3) is highly reactive and hence possesses low stability. The half-life of retinoic acid is approximately 1 h, which is in part due to CYP metabolism performing hydroxylation [83]. There are several known isoforms of retinoic acid. The most common retinoic acid with physiological activity is an all-trans-retinoic acid [84]. There are two other well-known isoforms, such as 9-cis-retinoic acid and 13-cis-retinoic acid (Figure 4).
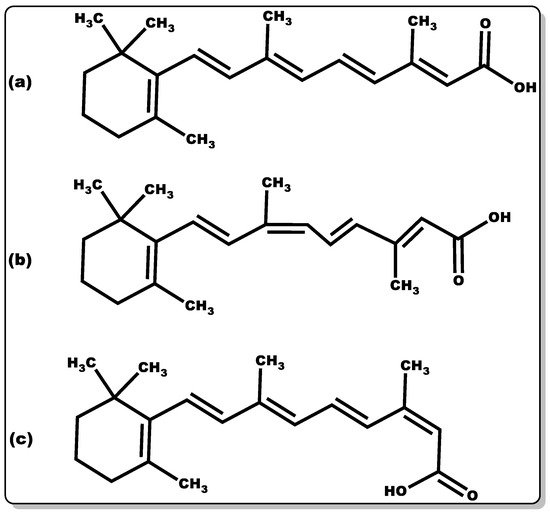
Figure 4. Molecular structure of retinoic acid in various isoforms: (a) all-trans-retinoic acid, (b) 9-cis-retinoic acid, and (c) 13-cis-retinoic acid.
Before the retinoic acid can bind to the receptors, to elicit the desired effect, they must first be transported to the correct location within the cell. Cellular retinoic acid-binding proteins (CRABPs) bind to the all-trans-retinoic acid, with high affinity, and can then be transported into the nucleus [84].
Retinoic acids mainly regulate gene expression via interaction with both nuclear and cytosolic receptors [59]. There are specific retinoic acid receptors (RARs) that are important regulators for development. There are three characterised RAR-coding genes: -α, -β, and -γ, and retinoid X receptors (RXRs). These receptors are expressed in fibroblasts and keratinocytes, and the expression of these receptors is even regulated by retinoic acid [84]. Retinoic acid can block collagenase activity, the enzymes that break down collagen, hence preventing collagen degradation [75]. Retinoic acid can regulate gene expression in both the epidermis and dermis. The genes are modulated concerning translation, transcription factors, RNA metabolism, receptor expression, and apoptosis. All-trans-retinoic acid is used in the treatment of skin cancer and acute promyelocytic leukaemia (APL). Conversely, a deficiency of retinoic acid has been associated with cancer progression and various dermatological diseases [84].
Similar to retinol, retinoic acid is also commonly used to treat acne and wrinkles/ageing [84]. This is due to the impact of retinoic acid on increased epithelial cell differentiation and proliferation, as well as the proliferation of keratinocytes and fibroblasts [59].
All trans-retinoic acid has been shown to have fungistatic effects, which can be used for psoriasis patients possessing a predisposition to fungal infections [85].
4. Vitamin C
Vitamin C (VTC, also known as ascorbic acid (Figure 5)) is a hydrophilic molecule with potent pharmacological and physiological activities, including antioxidant, anti-inflammatory, antimicrobial, and wound healing efficacy [86][87][88][89]. Plant sources are known to possess an abundant amount of VTC, including vegetables and fruits. Traditionally, they have been explored as antioxidants in food supplements, preservatives [90], and the management or prevention of scurvy [87]. The immunomodulatory activity of VTC in influencing the signalling pathway for cell differentiation and proliferation is well documented [91][92][93]. VTC is a gluconic acid lactone obtained from glucuronic acid and hydrophilic keto-lactone having two ionisable hydroxyl moieties [94]. VTC mostly exists in two equal enantiomers, including D-ascorbic acid and L-ascorbic acid which are mutually interchangeable [94]; however, the most common and bioactive isomer of VTC is L-ascorbic acid.
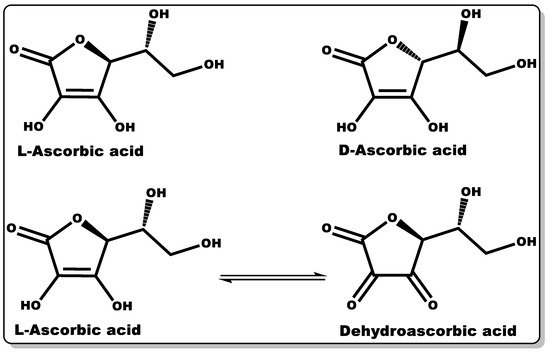
Figure 5. Molecular structures of ascorbic acid and its oxidised form.
Dehydroascorbic acid (DHAA) is an oxidised form of ascorbic acid (AA), and it can be converted to AA in the presence of a reducing agent [94]. AA and DHAA have been applied as active ingredients in cosmetic formulations and antimicrobial agents in pharmaceutical products [95][96][97][98][99], especially in skin tanning and the treatment or prevention of gingivitis, respectively [95][96][97]. Numerous studies have demonstrated the antimicrobial activity of VTC against both Gram-negative and Gram-positive bacteria, including S. mutans, P. gingivalis, S. aureus, H. pylori, B. subtilis, and M. tuberculosis, and fungi including C. albicans, Aspergillus niger, and A. flavus [100][101][102][103][104][105]. In a dose-dependent study by Verghese and co-workers, VTC was found to inhibit the growth of uropathogenic Escherichia coli and K. pneumoniae at a MIC value of 1% [100]. Moreover, Isela et al. demonstrated MIC values of VTC against S. mutans, S. aureus, P. gingivalis, C. albicans, and E. faecalis and their biofilms of 1% and 2%, respectively [103]. According to Mousavi et al., the MIC of VTC against C. jejuni-infected mice was found to be 0.1409% at pH 7.3 [88]. Moreover, a number of studies have demonstrated the disruption of bacterial biofilms at low VTC concentrations [104][106]. The prevention or inhibition of bacterial biofilm formation by VTC is attributed to its bacterial anti-quorum-sensing properties and the disruption of extracellular polymeric substance (EPS) biosynthesis. The EPS matrix is mainly made up of polysaccharides, proteins, and extracellular DNA, which affords defence to bacterial biofilms against host immunity and antibiotics from attacking bacteria [88][107]. This antibiofilm-formation property of VTC could be explored with other molecules (antibiotics) that can directly attack and eliminate AMR recalcitrant planktonic bacteria, including P. aeruginosa and MRSA [88].
VTC is innocuous against skin cells, making it suitable for topical cosmetical formulations [98][99]. The physiological role of VTC is imperative due to its crucial activity in skin fibroblast growth and migration, as well as the production of collagen and elastin, which are vital for wound healing or contraction [108][109][110]. It also possesses the capability to prevent changes associated with photoageing [92][93][110]. The wound healing efficacy of VTC was proven by Bikkera et al., where they investigated the impact of AA on wound healing in surgical patients, and it was found that AA deficiency impairs wound healing [86]. The wound care efficacy of VTC is attributed to its antioxidant, anti-inflammatory, antimicrobial, and collagen synthesis properties [86][87][89][110]. Several studies have shown the anti-inflammatory activity of VTC, and this follows its capacity for the downregulation of proinflammatory cytokines causing persistent inflammation [88][111].
A report by Lykkesfeldt et al. demonstrated the capability of VTC in the regeneration of vitamin E (tocopherols) from its oxidised form (tocopheroxyl radical), thereby affording VTC to indirectly inhibit lipid peroxidation [87]. Moreover, the combination of VTC and vitamin E has been demonstrated to afford maximum photoprotection of the skin, thereby limiting photoageing [112][113][114][115][116].
5. Vitamins E
Vitamin E (VTE, also known as tocochromanol) is composed of two major hydrophobic low-molecular-weight compounds grouped as tocopherols and tocotrienols. Tocopherols and tocotrienols are structurally identical with their chromanol rings (Figure 6), but have differences in their side chain, with the former having a long, saturated chain (phytyl) and the latter showing an unsaturated chain (farnesyl) with double bonds at positions 3′, 7′, and 11′, [117].
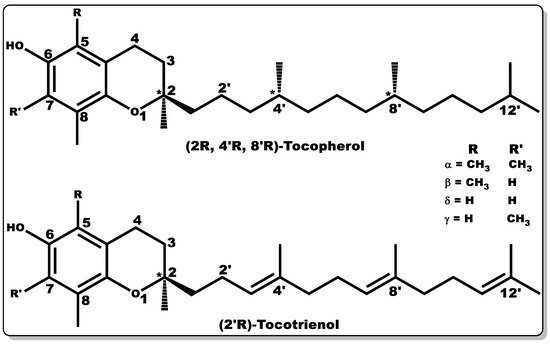
Figure 6. Molecular structures of tocochromanols (tocopherol and tocotrienol). Various isoforms of tocochromanols vary at position 5 or 7 (R or R’) of the chromanol ring with either -H or -CH3 moieties. In addition, the side chain of both tocochromanols differ, with tocopherol having a saturated chain while tocotrienol possess unsaturated side chain (double bond) at positions 3′, 7′, and 11′.
Both tocochromanols have eight subgroups, with each group accounting for four “isomers” each, existing as alpha (α), beta (β), delta (δ), and gamma (γ) [117][118][119]. VTE possesses anti-inflammatory, antioxidant, antibacterial, and wound-healing properties [117][119][120][121][122][123][124]. Furthermore, vitamin E is capable of preventing biofilm formation [125]. VTE is mostly obtained from natural sources, including plant seeds, nuts, corn, soybean, fruits, and vegetables [118][119][126][127][128]. α–Tocopherol is the major vitamin E component in humans with bioactivity [119][129][130], and its regulation of metabolic processes has been well documented [119][120][122][123]. α–Tocopherol has been used as a dietary supplement and as a component of skincare formulations [131][132].
Tocopherols are lipid-soluble molecules with ease of skin penetrability due to their low molecular weight, and they have been applied when alone or in combination with other molecules. Their antioxidant activity is devoid of skin irritation and they are capable of inhibiting allergic epidermal reactions, making them suitable for topical application [93][133][134]. In a study by Kuriyama et al., the topical administration of tocopherol was found to inhibit the irritation and allergic reaction often associated with contact dermatitis by regulating the keratinocytes [133]. Its antioxidant activity functions by transferring hydrogen to free radicals, including peroxyl, oxygen, and superoxide anions, thereby scavenging the radicals, affording protection to polyunsaturated fatty acids (PUFAs) from oxidation, inhibition of lipid peroxidation, and reduction of the skin ageing rate [134][135]. Moreover, tocopherol has been reported to have the capacity to regulate T-cell proliferation and interleukin-2 generation [124][136][137][138]. Furthermore, it has been shown to serve as an enzyme activity modulator, including protein kinase C (PKC), responsible for cell-mediated immune responses and cell proliferation, such as smooth muscle growth. It plays a role in the deactivation of PKC by inhibiting smooth muscle growth [137][139][140]. Tocopherols have been shown to possess potent biological activity in preventing infectious diseases [138]. Tocopherol by itself or when combined with antibiotics has demonstrated antibacterial activity [124][141]; however, its interaction with other molecules has been proven to have broad applicability [142][143]. To obtain the water-soluble or amphiphilic form of tocopherol, the esterification of the tocopherol derivative (D-α-tocopheryl succinate) with polyethene glycol 1000 results in D-α-tocopheryl polyethene glycol 1000 succinate (TPGS) (Figure 7) [144]. TPGS, a hydrophilic form of tocopherol, is made up of a lipophilic α-tocopherol and a hydrophilic PEG chain [144].
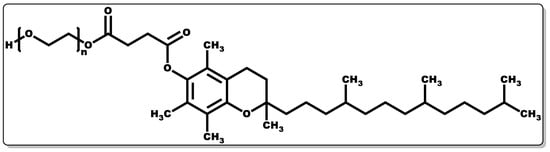
Figure 7. Structure of α-tocopheryl polyethene glycol 1000 succinate.
Studies have shown that TPGS has high bioavailability in comparison to hydrophilic tocopherol formulations in children with chronic cholestasis, indicating the potential of TPGS to serve as an alternative to tocopherol in order to avoid the injection of vitamin E formulations in chronic cholestasis [144][145]. TPGS is generally classified by USFDA as a safe substance, which has given it applicability in the pharmaceutical industry as an adjuvant to enhance drug molecules’ solubility, absorptivity, stability, and bioavailability [144]. A number of studies have shown the improvement in the oral absorptivity of vancomycin hydrochloride and talinolol in animals when in formulation with TPGS [146][147]. TPGS has been successfully used as a nano-vehicle for the delivery of drug molecules with low solubility and poor permeability [142][143]. A known example is cisplatin, a potent antineoplastic agent with poor hydrophilicity; however, upon combination with TPGS, there was a remarkable improvement in its physicochemical disposition [142][143]. TPGS has been reported to possess antitumorigenic activity when alone and in combination with other drug molecules, and this is evidenced by its improved pharmacological response in formulation with cisplatin [142][143]. Vitamin E or TPGS have been reported to synergistically elicit antibacterial activity when combined with other molecules (e.g., antibiotics) by downregulating efflux pump gene expression, leading to the lowering of the bacterial efflux pump activity, allowing the effective dose of antibiotics to reach the target bacterial cells [124][141][148]. Moreover, vitamin E or TPGS can enhance the penetration of antibiotics into bacterial cells, making them a suitable pharmaceutical adjuvant for antibiotics [149]. There is growing research demonstrating that other forms of VTE possess similar or superior biological activity in comparison to α-tocopherol [150]. In particular, the superior functionality of tocotrienols results in more effective penetration and distribution in the lipid layers of the cell membrane due to their unsaturated side chains having a higher affinity for the saturated lipid layers of biological tissues, including the brain and liver [150][151][152]. Tocopherols and tocotrienols (Figure 6) only differ in their side chains, with the latter having double bonds (unsaturated) at positions 3′, 7′, and 11′, as mentioned earlier. However, both have four different forms each, often classified as α, β, δ, and γ [152][153]. For instance, tocotrienols have been reported to exhibit superior antioxidant, analgesic, anti-inflammatory, antibacterial, anti-cancer, neuroprotective, and cholesterol modulation properties in comparison to those demonstrated by tocopherols [117][150][152]. Studies by Pearce and co-workers demonstrated the efficacy of tocotrienol at micromolar concentrations in inhibiting the enzyme in the liver (HMG-CoA reductase) responsible for the synthesis of cholesterol [154][155].
Overall, both tocopherols and tocotrienols possess significant biological activities, including antioxidant, anti-inflammatory, and antibacterial dispositions, which could be responsible for their wound healing efficacy. As demonstrated by several researchers, the wound healing efficacy of tocochromanols, when combined with antibiotics, is quite profound for preclinical data with clinical potential in humans [117][156][157]. In many studies, the oral and topical administration of tocochromanols was found to elicit wound healing efficacy. All of the findings regarding tocochromanols, when alone or in formulation with other molecules, demonstrated them fostering angiogenesis, epithelisation, granulation, and collagen production, accounting for rapid wound contraction and tissue regeneration [121][158][159][160][161][162].
6. Chitosan
Chitosan (CTN, Figure 8) is a biocompatible linear amino polysaccharide consisting of glucosamine and N-acetyl glucosamine units connected through β-(1→4) glycosidic bonds [163][164]. CTN is obtained from chitin, which is mostly found in crustaceans and shellfish. The versatility of CTN has earned it applicability in several industries, including medicine, pharmaceutical, cosmetics, agrochemistry, food, and beverage [163][164].
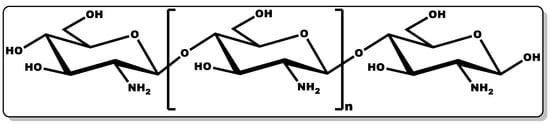
Figure 8. Structure of chitosan.
CTN pharmaceutical and medical applications have demonstrated its pharmacological and physiological roles, including its antioxidant, anti-inflammatory, antimicrobial, and wound healing efficacy [163][165][166][167][168][169][170]. Its potency in fostering chronic wound healing has been explored in various forms, including as powders, hydrogels, sponges, nanoparticles, bandages, and films [163][170]. Several authors have reported the antimicrobial and wound healing efficacy of CTN when incorporated in bandages alone or in combination with antibiotics [170][171][172][173]. This was proven when bandages containing only CTN were administered on bleeding wounds, leading to the rapid inhibition of haemorrhaging [163][170][171][172][173][174]. This observation could be attributed to the positively charged polysaccharide amine of CTN attracting negatively charged red blood cells (RBC), fostering blood clotting through the electrostatic interaction of the CTN and RBC [163][170][171][172][173]. In a study by Nimal et al., they demonstrated the remarkable efficacy of CTN bandages containing antibiotics with sustained release of the antibiotics for two weeks, leading to a significant reduction in the bacterial loads of the various polymicrobial cultures tested, including C. albicans, E. coli, and S. aureus [165]. In another study by Marangon et al., it was further established that the incorporation of CTN with rhamnolipid not only improved the antibacterial activity of the antibiotic agent against diverse strains of Staphylococcus, but also stabilised the CTN, showing the effective synergy between the two molecules [166]. Furthermore, studies by several authors have demonstrated the effectiveness of CTN in promoting tissue remodelling, with a reduction of scar tissue and an increase in the wound healing efficacy [163][167][168][169][170][174][175]. This is evidenced by a study conducted by Baxter and co-workers, in which a chitosan dressing was applied to a third-degree burn (mice model) leading to wound contraction [167]. The chitosan modulation of transforming growth factor-β1 (TGF-β1) and collagen III deposition in the wounds facilitated tissue remodelling and a subsequent reduction in TGF-β1, preventing the formation of a scar at the wound site. This is coupled with the recruitment of fibroblasts and the inhibition of inflammatory cytokines release, affording limited-pain wound healing [167]. Moreover, antimicrobial and wound healing study conducted by Dai et al. on mice infected burn, demonstrated the efficacy of CTN in the rapid bactericidal activity against pathogenic bacteria whilst promoting wound recovery [174].
CTN works by modulating the various cellular processes involved in wound healing by reducing the microbial loads and regulating growth factor expression (such as epidermal growth factor and TGF-β1) during wound healing phases [165][166][167][168]. In chronic wounds, CTN is thought to reduce the bacterial load by inhibiting or eliminating polymicrobial growth in infectious wounds. This antibacterial activity of CTN is achieved when the positively charged component of CTN interfaces with the negatively charged component of the bacterial cell membrane [165][166][167][168]. This coherence results in the inhibition of the bacterial cell membrane’s protein biosynthesis and translation. CTN is efficacious against both Gram-positive and Gram-negative bacteria [168]. However, it is more potent against Gram-negative bacteria, and this is adduced to the highly negatively charged envelope that the cell wall of Gram-negative bacteria possesses, which has a greater affinity for the positively charged polysaccharide amine group of CTN [168]. Furthermore, it fosters the efficient migration of neutrophils with the subsequent proliferation of fibroblasts. This is followed by its facilitation of macrophages and neutrophil infiltration and migration at the wound site, leading to the elimination of extraneous matters and promotion of granulation/fibrous tissue and re-epithelialisation. Its antimicrobial characteristics make it ideal for the prevention of wounds’ microbial infection or the inhibition of microbial growth in infected wounds [163][165][166][168][174]. Moreover, the tissue regeneration capacity of CTN is essential for wound contraction and re-epithelialisation [163][167][168][169][170][171][174]. CTN has been explored alone and in combination with other molecules or as a pharmaceutical excipient [163][164][176], and it is well tolerated and biocompatible [137][177][178]. CTN could serve as an ideal gelling agent and adjuvant for the controlled release of active ingredients for topical cosmetic formulations due to its biocompatibility, biodegradability, and compatibility with other cosmetic active ingredients, including vitamins [163][171][179][180].
7. Aloe vera
Aloe vera, from the Liliaceae family, has proven pharmacological activities against dry skin, burns, acne, psoriasis, and wounds [181][182][183][184]. It has been well applied in several industrial applications, including cosmetics, food, and beverages. Its use in cosmetic topical application could be attributed to its moisturising and soothing effect [185][186]. The phytoconstituents of Aloe vera include water, vitamins (A–C and E), minerals (Na, K, Fe, and Zn), phenolics, and amino acids (folic acid). Interestingly, these components have been demonstrated to possess therapeutic activity, such as antimicrobial, anti-inflammatory, and wound healing [181][182][183][184]. Reports have exhibited the antibacterial disposition of Aloe vera against both Gram-positive and Gram-negative bacteria, with MIC of ≤ 0.000625% for Pseudomonas aeruginosa, Bacillus subtilis, and ≤0.005% for Staphylococcus aureus [187]. In another study by Goudarzi et al., Aloe vera was found to be efficacious against P. aeruginosa strains from burn wounds with an MIC value of 0.02% [188]. The antibacterial activity of A. vera may be due to its anthraquinone phytoconstituents [187][188]. Furthermore, the anti-inflammatory and wound healing efficacy of A. vera has been shown by many reports [183][184][189]. The preventive and healing effect of A. vera against pressure ulcers was demonstrated by Hekmatpou and co-workers, where they carried out a randomised triple-blind clinical trial, and it was observed that A. vera was capable of preventing or fostering the healing of pressure ulcers by modulating the wound’s temperature, non-blanchable redness, swelling, and pain [184]. Numerous studies have demonstrated the efficacy of A. vera in the modulation of proinflammatory cytokine gene expression, a known promoter of IL-6, NO, causing persistent inflammation [183][184][189]. This inhibition has been attributed to reduced inflammatory reaction and rapid wound healing [183][184]. Moreover, A. vera has been shown to possess tissue regeneration disposition by fostering fibroblast proliferation with collagen biosynthesis [184].
8. Cinnamaldehyde
Cinnamaldehyde (CME, Figure 9) is a phenylpropanoid molecule obtained from the bark of cinnamon trees with proven therapeutic action, including antimicrobial, anti-inflammatory, and wound healing efficacy [190][191][192][193].
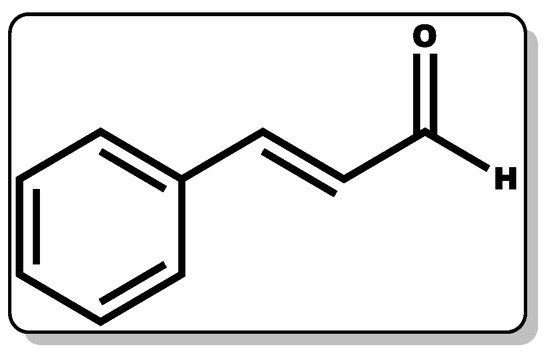
Figure 9. Molecular structure of cinnamaldehyde.
It has been found to be useful in the beverage, cosmetic, and agrochemical industries [190][194][195]. CME has exhibited activity in the inhibition or elimination of pathogenic fungi, including Candida albicans and Aspergillus flavus [192][193]. Moreover, this compound has been shown to repel insects, kill or inhibit certain bacterial growth, and prevent biofilm formation [107][195]. Several studies have demonstrated the antimicrobial properties of cinnamaldehyde, including pathogenic Gram-positive and Gram-negative bacteria, such as P. aeruginosa, E. coli, and S. aureus [107][196]. According to Ramasamy and co-workers, the efficacy of CME against P. aeruginosa, E. coli, and S. aureus is limited at concentrations ranging from 0.0005% to 0.025%; however, on incorporation with nanoparticles, the MIC and MBC were greatly improved, inferring the synergistic disposition between CME and other molecules. In addition, in a study conducted by Topa et al., it was reported that CME with MIC (0.16%) was found to elicit bacteriostatic action against P. aeruginosa [107]. Another study by Utchariyakiat and co-workers showed that the MIC of CME against P. aeruginosa ranged from 0.0562% to 0.225% [197]. A recent study by Pereira and co-workers showed that CME is potent against E. coli at a MIC of 0.078% [198]. In a similar study, it was established that CME was well tolerated by human epithelial cells [198]. The antimicrobial action of CME may be by disrupting the cellular homeostasis of the bacterial cell membrane, thereby impeding its growth [198]. Moreover, CME has been shown to possess an anti-inflammatory disposition, which is essential for wound management [199]. The antibacterial and anti-inflammatory dispositions of CME are useful in the management of wounds due to its capacity to eliminate or prevent bacterial biofilms (P. aeruginosa) and its reduction of the inflammatory reaction by inhibiting high-influx of inflammatory infiltrates. Moreover, numerous reports have demonstrated that CME is capable of accelerating collagen production and the induction of mammalian endothelial cell growth, which is crucial for wound healing [191][200][201]. This was demonstrated in a study by Ferro et al. where CME was tested against P. aeruginosa-infected mice skin wounds, and it was observed that the bacterium metabolic rate and its ability to cause biofilm formation was reduced at sub-inhibitory concentrations of CME. Furthermore, routine topical administration of CME was reported to have lowered the bacterium bioburden of the mice’s skin wounds with rapid wound contraction and healing. Further analysis showed that the CME-treated wound samples had lower interleukin-17, vascular endothelial growth factor, and nitric oxide levels compared to the untreated wound samples [201]. The modulation of these inflammatory infiltrates by CME may have contributed to its wound healing action.
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics14051021
References
- Chattopadhyay, I.; Biswas, K.; Bandyopadhyay, U.; Banerjee, R.K. Turmeric and Curcumin: Biological Actions and Medicinal Applications. Curr. Sci. 2004, 87, 44–53. Available online: http://www.jstor.org/stable/24107978 (accessed on 10 February 2022).
- Sharma, R.A.; Steward, W.P.; Gescher, A.J. Pharmacokinetics and pharmacodynamics of curcumin. Adv. Exp. Med. Biol. 2007, 595, 453–470.
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2016, 7, 205–233.
- Edwards, R.L.; Luis, P.B.; Varuzza, P.V.; Joseph, A.I.; Presley, S.H.; Chaturvedi, R.; Schneider, C. The anti-inflammatory activity of curcumin is mediated by its oxidative metabolites. J. Biol. Chem. 2017, 292, 21243–21252.
- Kim, H.; Ban, I.; Choi, Y.; Yu, S.; Youn, S.J.; Baik, M.-Y.; Lee, H.; Kim, W. Puffing of Turmeric (Curcuma longa L.) Enhances its Anti-Inflammatory Effects by Upregulating Macrophage Oxidative Phosphorylation. Antioxidants 2020, 9, 931.
- Meng, B.; Li, J.; Cao, H. Antioxidant and anti-inflammatory activities of curcumin on diabetes mellitus and its complications. Curr. Pharm. Des. 2013, 19, 2101–2113.
- Aggarwal, S.; Ichikawa, H.; Takada, Y.; Sandur, S.K.; Shishodia, S.; Aggarwal, B.B. Curcumin (Diferuloylmethane) Down-Regulates Expression of Cell Proliferation and Antiapoptotic and Metastatic Gene Products through Suppression of IκBα Kinase and Akt Activation. Mol. Pharmacol. 2006, 69, 195–206.
- Ono, M.; Higuchi, T.; Takeshima, M.; Chen, C.; Nakano, S. Differential anti-tumor activities of curcumin against Ras- and Src-activated human adenocarcinoma cells. Biochem. Biophys. Res. Commun. 2013, 436, 186–191.
- Chuengsamarn, S.; Rattanamongkolgul, S.; Luechapudiporn, R.; Phisalaphong, C.; Jirawatnotai, S. Curcumin Extract for Prevention of Type 2 Diabetes. Diabetes Care 2012, 35, 2121–2127.
- Chen, T.-Y.; Chen, D.-Y.; Wen, H.-W.; Ou, J.-L.; Chiou, S.-S.; Chen, J.-M.; Wong, M.-L.; Hsu, W.-L. Inhibition of Enveloped Viruses Infectivity by Curcumin. PLoS ONE 2013, 8, e62482.
- Jennings, M.; Parks, R. Curcumin as an Antiviral Agent. Viruses 2020, 12, 1242.
- Mun, S.-H.; Joung, D.-K.; Kim, Y.-S.; Kang, O.-H.; Kim, S.-B.; Seo, Y.-S.; Kim, Y.-C.; Lee, D.-S.; Shin, D.-W.; Kweon, K.-T.; et al. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine 2013, 20, 714–718.
- Teow, S.-Y.; Liew, K.; Ali, S.A.; Khoo, A.S.-B.; Peh, S.-C. Antibacterial Action of Curcumin against Staphylococcus aureus: A Brief Review. J. Trop. Med. 2016, 2016, 2853045.
- Jiang, Y.; Leung, A.W.; Hua, H.; Rao, X.; Xu, C. Photodynamic Action of LED-Activated Curcumin against Staphylococcus aureus Involving Intracellular ROS Increase and Membrane Damage. Int. J. Photoenergy 2014, 2014, 637601.
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153.
- Shlar, I.; Droby, S.; Choudhary, R.; Rodov, V. The mode of antimicrobial action of curcumin depends on the delivery system: Monolithic nanoparticles vs. supramolecular inclusion complex. RSC Adv. 2017, 7, 42559–42569.
- Kaur, S.; Modi, N.H.; Panda, D.; Roy, N. Probing the binding site of curcumin in Escherichia coli and Bacillus subtilis FtsZ—A structural insight to unveil antibacterial activity of curcumin. Eur. J. Med. Chem. 2010, 45, 4209–4214.
- Yun, D.G.; Lee, D.G. Antibacterial activity of curcumin via apoptosis-like response in Escherichia coli. Appl. Microbiol. Biotechnol. 2016, 100, 5505–5514.
- Di Nardo, V.; Gianfaldoni, S.; Tchernev, G.; Wollina, U.; Barygina, V.; Lotti, J.; Daaboul, F.; Lotti, T. Use of Curcumin in Psoriasis. Open Access Maced. J. Med Sci. 2018, 6, 218–220.
- Vollono, L.; Falconi, M.; Gaziano, R.; Iacovelli, F.; Dika, E.; Terracciano, C.; Bianchi, L.; Campione, E. Potential of Curcumin in Skin Disorders. Nutrients 2019, 11, 2169.
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent Developments in Delivery, Bioavailability, Absorption and Metabolism of Curcumin: The Golden Pigment from Golden Spice. Cancer Res. Treat. 2014, 46, 2–18.
- Wang, Y.-J.; Pan, M.-H.; Cheng, A.-L.; Lin, L.-I.; Ho, Y.-S.; Hsieh, C.-Y.; Lin, J.-K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876.
- Esatbeyoglu, T.; Ulbrich, K.; Rehberg, C.; Rohn, S.; Rimbach, G. Thermal stability, antioxidant, and anti-inflammatory activity of curcumin and its degradation product 4-vinyl guaiacol. Food Funct. 2015, 6, 887–893.
- Anand, P.; Nair, H.B.B.; Sung, B.; Kunnumakkara, A.B.; Yadav, V.R.; Tekmal, R.R.; Aggarwal, B.B. Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem. Pharmacol. 2010, 79, 330–338.
- Schiborr, C.; Kocher, A.; Behnam, D.; Jandasek, J.; Toelstede, S.; Frank, J. The oral bioavailability of curcumin from micronized powder and liquid micelles is significantly increased in healthy humans and differs between sexes. Mol. Nutr. Food Res. 2014, 58, 516–527.
- Comotto, M.; Saghazadeh, S.; Bagherifard, S.; Aliakbarian, B.; Kazemzadeh-Narbat, M.; Sharifi, F.; Shaegh, S.A.M.; Arab-Tehrany, E.; Annabi, N.; Perego, P.; et al. Breathable hydrogel dressings containing natural antioxidants for management of skin disorders. J. Biomater. Appl. 2019, 33, 1265–1276.
- Zheng, D.; Huang, C.; Huang, H.; Zhao, Y.; Khan, M.R.U.; Zhao, H.; Huang, L. Antibacterial Mechanism of Curcumin: A Review. Chem. Biodivers. 2020, 17, e202000171.
- Mehrabani, D.; Farjam, M.; Geramizadeh, B.; Tanideh, N.; Amini, M.; Panjehshahin, M.R. The healing effect of cur-cumin on burn wounds in rat. World J. Plast. Surg. 2015, 4, 29–35.
- Sidhu, G.S.; Singh, A.K.; Thaloor, D.; Banaudha, K.K.; Patnaik, G.K.; Srimal, R.C.; Maheshwari, R.K. Enhancement of wound healing by curcumin in animals. Wound Repair Regen. 1998, 6, 167–177.
- Sharma, M.; Sahu, K.; Singh, S.P.; Jain, B. Wound healing activity of curcumin conjugated to hyaluronic acid: In vitro and in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1009–1017.
- Miah, A.H.; Hasan, M.; Sarker, Y.; Alam, M.; Juyena, N. Clinical evaluation of ethanolic extract of curcumin (Curcuma longa) on wound healing in Black Bengal goats. J. Adv. Vet. Anim. Res. 2017, 4, 181–186.
- Jagetia, G.C.; Rajanikant, G.K. Curcumin Stimulates the Antioxidant Mechanisms in Mouse Skin Exposed to Fractionated γ-Irradiation. Antioxidants 2015, 4, 25–41.
- Hegge, A.B.; Andersen, T.; Melvik, J.E.; Bruzell, E.; Kristensen, S.; Tønnesen, H.H. Formulation and Bacterial Phototoxicity of Curcumin Loaded Alginate Foams for Wound Treatment Applications: Studies on Curcumin and Curcuminoides XLII. J. Pharm. Sci. 2011, 100, 174–185.
- Chindera, K.; Mahato, M.; Sharma, A.K.; Horsley, H.; Kloc-Muniak, K.; Kamaruzzaman, N.F.; Kumar, S.; McFarlane, A.; Stach, J.; Bentin, T.; et al. The antimicrobial polymer PHMB enters cells and selectively condenses bacterial chromosomes. Sci. Rep. 2016, 6, 2312.
- Gray, D.; Barrett, S.; Battacharyya, M.; Butcher, M.; Enoch, S.; Fumerola, S.; Stephen-Haynes, J.; Edwards-Jones, V.; Leaper, D.; Strohal, R.; et al. PHMB and its potential contribution to wound management. Wounds 2010, 6, 40–46.
- Consensus Panel. PHMB and Its Potential Contribution to Wound Management. A Consensus Document. Wounds. 2010, pp. 1–16. Available online: https://www.wounds-uk.com/resources/details/consensus-document-phmb-and-its-potential-contribution-wound-management (accessed on 10 February 2022).
- Valluri, S.; Fleming, T.P.; Laycock, K.A.; Tarle, I.S.; Goldberg, M.A.; Garcia-Ferrer, F.J.; Essary, L.R.; Pepose, J.S. In Vitro and In Vivo Effects of Polyhexamethylene Biguanide against Herpes Simplex Virus Infection. Cornea 1997, 16, 556–559.
- Gentile, A.; Gerli, S.; Di Renzo, G.C. A new non-invasive approach based on polyhexamethylene biguanide increases the regression rate of HPV infection. BMC Clin. Pathol. 2012, 12, 17.
- Zheng, Y.; Wang, D.; Ma, L. Effect of Polyhexamethylene Biguanide in Combination with Undecylenamidopropyl Betaine or PslG on Biofilm Clearance. Int. J. Mol. Sci. 2021, 22, 768.
- Gilbert, P.; Moore, L.E. Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 2005, 99, 703–715.
- Sanada, H.; Nakagami, G.; Takehara, K.; Goto, T.; Ishii, N.; Yoshida, S.; Ryu, M.; Tsunemi, Y. Antifungal Effect of Non-Woven Textiles Containing Polyhexamethylene Biguanide with Sophorolipid: A Potential Method for Tinea Pedis Prevention. Healthcare 2014, 2, 183–191.
- Kim, S.Y.; Hahn, T.W.; Kong, H.H.; Chung, D.; Hahn, Y.H. In Vitro Amoebicidal Efficacy of Hexamidine, Polyhexa-Methylene Biguanide and Chlorhexidine on Acanthamoeba Ocular Isolates. J. Korean Ophthalmol. Soc. 1999, 40, 933–940. Available online: https://www.jkos.org/journal/view.php?number=3878 (accessed on 10 February 2022).
- Dart, J. Polyhexamethylene Biguanide (PHMB) Ophthalmic Solution in Subjects Affected by Acanthamoeba Keratitis, 23/10/2020 ClinicalTrials.gov Identifier: NCT03274895. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT03274895 (accessed on 20 January 2022).
- European Chemical Agency (ECHA). Polyaminopropyl Biguanide (PHMB), Final Opinion on Polyaminopropyl Bigua-nide (PHMB)—Submission III. 2017. Available online: https://ec.europa.eu/health/sites/default/files/scientific_committees/consumer_safety/docs/sccs_o_204.pdf (accessed on 5 December 2021).
- Moore, K.; Gray, D. Using PHMB antimicrobial to prevent wound infection. Wounds 2007, 3, 96–102.
- Sowlati-Hashjin, S.; Carbone, P.; Karttunen, M. Insights into the Polyhexamethylene Biguanide (PHMB) Mechanism of Action on Bacterial Membrane and DNA: A Molecular Dynamics Study. J. Phys. Chem. B 2020, 124, 4487–4497.
- Kirker, K.R.; Fisher, S.T.; James, G.A.; McGhee, D.; Shah, C.B. Efficacy of Polyhexamethylene Biguanide-containing Antimicrobial Foam Dressing Against MRSA Relative to Standard Foam Dressing. Wounds 2009, 21, 229–233.
- Butcher, M. PHMB: An effective antimicrobial in wound bioburden management. Br. J. Nurs. 2012, 21, S16–S21.
- Daeschlein, G.; Assadian, O.; Bruck, J.; Meinl, C.; Kramer, A.; Koch, S. Feasibility and Clinical Applicability of Polihexanide for Treatment of Second-Degree Burn Wounds. Skin Pharmacol. Physiol. 2007, 20, 292–296.
- Fabry, W.; Trampenau, C.; Bettag, C.; Handschin, A.E.; Lettgen, B.; Huber, F.-X.; Hillmeier, J.; Kock, H.-J. Bacterial decontamination of surgical wounds treated with Lavasept®. Int. J. Hyg. Environ. Health 2006, 209, 567–573.
- Wiegand, C.; Abel, M.; Ruth, P.; Hipler, U.-C. HaCaT keratinocytes in co-culture with Staphylococcus aureus can be protected from bacterial damage by polihexanide. Wound Repair Regen. 2009, 17, 730–738.
- Mueller, S.W.; Krebsbach, L.E. Impact of an antimicrobial-impregnated gauze dressing on surgical site infections including methicillin-resistant Staphylococcus aureus infections. Am. J. Infect. Control 2008, 36, 651–655.
- Lenselink, E.; Andriessen, A. A cohort study on the efficacy of a polyhexanide-containing biocellulose dressing in the treatment of biofilms in wounds. J. Wound Care 2011, 20, 534–539.
- Alblas, J.; Andriessen, A.; Klicks, R.; Wiersema, A.; Doorn, J.; Elzinga, G.; Spits, H.; Post, A.; Gent, M. Clinical evaluation of a PHMBimpregnated biocellulose dressing on paediatric lacerations. J. Wound Care 2011, 20, 280–284.
- Hübner, N.-O.; Kramer, A. Review on the Efficacy, Safety and Clinical Applications of Polihexanide, a Modern Wound Antiseptic. Ski. Pharmacol. Physiol. 2010, 23, 17–27.
- Orfanos, C.; Zouboulis, C.P.D. Oral Retinoids in the Treatment of Seborrhoea and Acne. Dermatology 1998, 196, 140–147.
- Thielitz, A.; Gollnick, H. Topical Retinoids in Acne Vulgaris: Update on efficacy and safety. Am. J. Clin. Dermatol. 2008, 9, 369–381.
- Rusu, A.; Tanase, C.; Pascu, G.-A.; Todoran, N. Recent Advances Regarding the Therapeutic Potential of Adapalene. Pharmaceuticals 2020, 13, 217.
- Polcz, M.E.; Barbul, A. The Role of Vitamin A in Wound Healing. Nutr. Clin. Pract. 2019, 34, 695–700.
- Daly, T.J.; Weston, W.L. Retinoid effects on fibroblast proliferation and collagen synthesis in vitro and on fibrotic disease in vivo. J. Am. Acad. Dermatol. 1986, 15, 900–902.
- Wolbach, S.B.; Howe, P.R. Tissue changes following deprivation of fat-soluble a vitamin. J. Exp. Med. 1925, 42, 753–777.
- Varani, J.; Warner, R.L.; Gharaee-Kermani, M.; Phan, S.; Kang, S.; Chung, J.; Wang, Z.; Datta, S.C.; Fisher, G.J.; Voorhees, J.J. Vitamin A Antagonizes Decreased Cell Growth and Elevated Collagen-Degrading Matrix Metalloproteinases and Stimulates Collagen Accumulation in Naturally Aged Human Skin1. J. Investig. Dermatol. 2000, 114, 480–486.
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.-Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335–339.
- Sorg, O.; Iran, C.; Carraux, P.; Grand, D.; Hügin, A.; Didierjean, L.; Saurat, J.-H. Spectral Properties of Topical Retinoids Prevent DNA Damage and Apoptosis after Acute UV-B Exposure in Hairless Mice. Photochem. Photobiol. 2007, 81, 830–836.
- Sorg, O.; Antille, C.; Kaya, G.; Saurat, J.-H. Retinoids in cosmeceuticals. Dermatol. Ther. 2006, 19, 289–296.
- Törmä, H.; Brunnberg, L.; Vahlquist, A. Age-related variations in acyl-CoA: Retinol acyltransferase activity and vitamin A concentration in the liver and epidermis of hairless mice. Biochim. Biophys. Acta 1987, 921, 254–258.
- Antille, C.; Tran, C.; Sorg, O.; Carraux, P.; Didierjean, L.; Saurat, J.-H. Vitamin A Exerts a Photoprotective Action in Skin by Absorbing Ultraviolet B Radiation. J. Investig. Dermatol. 2003, 121, 1163–1167.
- Pechère, M.; Pechèreb, J.-C.; Siegenthalera, G.; Germaniera, L.; Saurata, J.-H. Antibacterial Activity of Retinaldehyde against Propionibacterium acnes. Dermatology 1999, 199, 29–31.
- Pechère, M.; Germanier, L.; Siegenthaler, G.; Pechère, J.-C.; Saurat, J.-H. The antibacterial activity of topical retinoids: The case of retinaldehyde. Dermatology 2002, 205, 153–158.
- Harris, T.A.; Gattu, S.; Propheter, D.C.; Kuang, Z.; Bel, S.; Ruhn, K.A.; Chara, A.L.; Edwards, M.; Zhang, C.; Jo, J.H.; et al. Resistin-like molecule α provides vitamin-A-dependent antimi-crobial protection in the skin. Cell Host Microbe 2019, 25, 777–788.
- Oliveira, L.D.M.; Teixeira, F.M.E.; Sato, M.N. Impact of Retinoic Acid on Immune Cells and Inflammatory Diseases. Mediat. Inflamm. 2018, 2018, 3067126.
- Anonymous. Isolation of Vitamin A. Nature 1932, 129, 88.
- Zasada, M.; Budzisz, E. Retinoids: Active molecules influencing skin structure formation in cosmetic and dermatological treatments. Postepy Dermatol. Allergol. 2019, 36, 392–397.
- Kafi, R.; Kwak, H.S.R.; Schumacher, W.E.; Cho, S.; Hanft, V.N.; Hamilton, T.A.; King, A.L.; Neal, J.D.; Varani, J.; Fisher, G.J.; et al. Improvement of Naturally Aged Skin with Vitamin A (Retinol). Arch. Dermatol. 2007, 143, 606–612.
- Kong, R.; Cui, Y.; Fisher, G.J.; Wang, X.; Chen, Y.; Schneider, L.M.; Majmudar, G. A comparative study of the effects of retinol and retinoic acid on histological, molecular, and clinical properties of human skin. J. Cosmet. Dermatol. 2016, 15, 49–57.
- Kang, S.; Duell, E.A.; Fisher, G.J.; Datta, S.C.; Wang, Z.-Q.; Reddy, A.P.; Tavakkol, A.; Yi, J.Y.; Griffiths, C.E.; Elder, J.T.; et al. Application of Retinol to Human Skin In Vivo Induces Epidermal Hyperplasia and Cellular Retinoid Binding Proteins Characteristic of Retinoic Acid but without Measurable Retinoic Acid Levels or Irritation. J. Investig. Dermatol. 1995, 105, 549–556.
- Duell, E.A.; Kang, S.; Elder, J.T.; Voorhees, J.J.; Derguini, F. Extraction of Human Epidermis Treated with Retinol Yields Retro-Retinoids in Addition to Free Retinol and Retinyl Esters. J. Investig. Dermatol. 1996, 107, 178–182.
- Brown, S.; Meroueh, S.; Fridman, R.; Mobashery, S. Quest for Selectivity in Inhibition of Matrix Metalloproteinases. Curr. Top. Med. Chem. 2004, 4, 1227–1238.
- Trengove, N.J.; Stacey, M.C.; Macauley, S.; Bennett, N.; Gibson, J.; Burslem, F.; Murphy, G.; Schultz, G. Analysis of the acute and chronic wound environments: The role of proteases and their inhibitors. Wound Repair Regen. 1999, 7, 442–452.
- Brandaleone, H.; Papper, E. The effect of the local and oral administration of cod liver oil on the rate of wound healing in vitamin a-deficient and normal rats. Ann. Surg. 1941, 114, 791–798.
- Ehrlich, H.P.; Hunt, T.K. Effects of Cortisone and Vitamin A on Wound Healing. Ann. Surg. 1968, 167, 324–328.
- Morton, R. Chemical Aspects of the Visual Process. Nature 1944, 153, 69–71.
- Ghyselinck, N.B.; Duester, G. Retinoic acid signaling pathways. Development 2019, 146, dev167502.
- Szymański, Ł.; Skopek, R.; Palusińska, M.; Schenk, T.; Stengel, S.; Lewicki, S.; Kraj, L.; Kamiński, P.; Zelent, A. Retinoic Acid and Its Derivatives in Skin. Cells 2020, 9, 2660.
- Campione, E.; Cosio, T.; Lanna, C.; Mazzilli, S.; Ventura, A.; Dika, E.; Gaziano, R.; Dattola, A.; Candi, E.; Bianchi, L. Predictive role of vitamin A serum concentration in psoriatic patients treated with IL-17 inhibitors to prevent skin and systemic fungal infections. J. Pharmacol. Sci. 2020, 144, 52–56.
- Bikker, A.; Wielders, J.; van Loo, R.; Loubert, M. Ascorbic acid deficiency impairs wound healing in surgical patients: Four case reports. Int. J. Surg. Open 2016, 2, 15–18.
- Lykkesfeldt, J.; Michels, A.J.; Frei, B. Vitamin C. Adv. Nutr. 2014, 5, 16–18.
- Mousavi, S.; Escher, U.; Thunhorst, E.; Kittler, S.; Kehrenberg, C.; Bereswill, S.; Heimesaat, M.M.; Mousavi, S.; Escher, U.; Thunhorst, E.; et al. Vitamin C alleviates acute enterocolitis in Campylobacter jejuni infected mice. Sci. Rep. 2020, 10, 2921.
- Otten, A.T.; Bourgonje, A.R.; Peters, V.; Alizadeh, B.Z.; Dijkstra, G.; Harmsen, H.J.M. Vitamin C Supplementation in Healthy Individuals Leads to Shifts of Bacterial Populations in the Gut—A Pilot Study. Antioxidants 2021, 10, 1278.
- Tajkarimi, M.; Ibrahim, S.A. Antimicrobial activity of ascorbic acid alone or in combination with lactic acid on Escherichia coli O157:H7 in laboratory medium and carrot juice. Food Control 2011, 22, 801–804.
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211.
- Wang, K.; Jiang, H.; Li, W.; Qiang, M.; Dong, T.; Li, H. Role of Vitamin C in Skin Diseases. Front. Physiol. 2018, 9, 819.
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Suliga, E. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203.
- Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Immunomodulatory and antimicrobial effects of vitamin C. Eur. J. Microbiol. Immunol. 2019, 9, 73–79.
- Ericsson, S.Y.; Stjernstrom, L.E. Anti-Infectant Topical Preparations. U.S. Patent 3,065,139, 20 November 1962. Available online: https://patents.google.com/patent/US3065139A/en (accessed on 15 February 2022).
- Fine, D. Gel Composition for Reduction of Gingival Inflammation and Retardation of Dental Plaque. U.S. Patent 5,298,237, 29 March 1994.
- Hovi, T.; Hirvimies, A.; Stenvik, M.; Vuola, E.; Pippuri, R. Topical treatment of recurrent mucocutaneous herpes with ascorbic acid-containing solution. Antivir. Res. 1995, 27, 263–270.
- Matsuda, S.; Shibayama, H.; Hisama, M.; Ohtsuki, M.; Iwaki, M. Inhibitory Effects of a Novel Ascorbic Derivative, Disodium Isostearyl 2-O-L-Ascorbyl Phosphate on Melanogenesis. Chem. Pharm. Bull. 2008, 56, 292–297.
- Maione-Silva, L.; De Castro, E.G.; Nascimento, T.L.; Cintra, E.R.; Moreira, L.C.; Cintra, B.A.S.; Valadares, M.C.; Lima, E.M. Ascorbic acid encapsulated into negatively charged liposomes exhibits increased skin permeation, retention and enhances collagen synthesis by fibroblasts. Sci. Rep. 2019, 9, 522.
- Verghese, R.J.; Mathew, S.K.; David, A. Antimicrobial activity of Vitamin C demonstrated on uropathogenic Escherichia coli and Klebsiella pneumoniae. J. Curr. Res. Sci. Med. 2017, 3, 88–93.
- Vilchèze, C.; Hartman, T.; Weinrick, B.; Jacobs, W.R., Jr. Mycobacterium tuberculosis is extraordinarily sensitive to killing by a vitamin C-induced Fenton reaction. Nat. Commun. 2013, 4, 1881.
- Zhang, H.M.; Wakisaka, N.; Maeda, O.; Yamamoto, T. Vitamin C inhibits the growth of a bacterial risk factor for gas-tric carcinoma: Helicobacter pylori. Cancer 1997, 80, 1897–1903.
- Isela, S.; Sergio, N.; Jose, M.; Rene, H.; Claudio, C. Ascorbic Acid on Oral Microbial Growth and Biofilm Formation. Pharma Innovation 2013, 2, 104–109. Available online: https://www.thepharmajournal.com/archives/?year=2013&vol=2&issue=4&ArticleId=179 (accessed on 23 January 2022).
- Pandit, S.; Ravikumar, V.; Abdel-Haleem, A.; Derouiche, A.; Mokkapati, V.R.S.S.; Sihlbom, C.; Mineta, K.; Gojobori, T.; Gao, X.; Westerlund, F.; et al. Low Concentrations of Vitamin C Reduce the Synthesis of Extracellular Polymers and Destabilize Bacterial Biofilms. Front. Microbiol. 2017, 8, 2599.
- Mumtaz, S.; Ali, S.; Tahir, H.M.; Kazmi, S.A.R.; Mughal, T.A.; Younas, M. Evaluation of antibacterial activity of vitamin C against human bacterial pathogens. Braz. J. Biol. 2021, 83, e247165.
- Majtan, J.; Sojka, M.; Palenikova, H.; Bucekova, M.; Majtan, V. Vitamin C Enhances the Antibacterial Activity of Honey against Planktonic and Biofilm-Embedded Bacteria. Molecules 2020, 25, 992.
- Topa, S.H.; Subramoni, S.; Palombo, E.A.; Kingshott, P.; Rice, S.A.; Blackall, L.L. Cinnamaldehyde disrupts biofilm formation and swarming motility of Pseudomonas aeruginosa. Microbiology 2018, 164, 1087–1097.
- Phillips, C.L.; Combs, S.B.; Pinnell, S.R. Effects of Ascorbic Acid on Proliferation and Collagen Synthesis in Relation to the Donor Age of Human Dermal Fibroblasts. J. Investig. Dermatol. 1994, 103, 228–232.
- Telang, P.S. Vitamin C in dermatology. Indian Dermatol. Online J. 2013, 4, 143–146.
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866.
- Ellulu, M.S.; Rahmat, A.; Ismail, P.; Khaza’Ai, H.; Abed, Y. Effect of vitamin C on inflammation and metabolic markers in hypertensive and/or diabetic obese adults: A randomized controlled trial. Drug Des. Dev. Ther. 2015, 2015, 3405–3412.
- Lin, J.-Y.; Selim, M.; Shea, C.R.; Grichnik, J.M.; Omar, M.M.; Monteiro-Riviere, N.; Pinnell, S.R. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J. Am. Acad. Dermatol. 2003, 48, 866–874.
- Burke, K.E. Interaction of vitamins C and E as better cosmeceuticals. Dermatol. Ther. 2007, 20, 314–321.
- Burke, K.E. Photoprotection of the Skin with Vitamins C and E: Antioxidants and Synergies. In Nutrition and Skin; Pappas, A., Ed.; Springer: New York, NY, USA, 2011; pp. 43–58.
- Eberlein-König, B.; Ring, J. Relevance of vitamins C and E in cutaneous photoprotection. J. Cosmet. Dermatol. 2005, 4, 4–9.
- Lintner, K.; Gerstein, F.; Solish, N. A serum containing vitamins C & E and a matrix-repair tripeptide reduces facial signs of aging as evidenced by Primos® analysis and frequently repeated auto-perception. J. Cosmet. Dermatol. 2020, 19, 3262–3269.
- Wong, S.K.; Kamisah, Y.; Mohamed, N.; Muhammad, N.; Masbah, N.; Fahami, N.A.M.; Mohamed, I.N.; Shuid, A.N.; Saad, Q.M.; Abdullah, A.; et al. Potential Role of Tocotrienols on Non-Communicable Diseases: A Review of Current Evidence. Nutrients 2020, 12, 259.
- Ng, M.H.; Choo, Y.M.; Ma, A.N.; Chuah, C.H.; Hashim, M.A. Separation of vitamin E (tocopherol, tocotrienol, and tocomonoenol) in palm oil. Lipids 2004, 39, 1031–1035.
- Jiang, Q. Natural forms of vitamin E: Metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014, 72, 76–90.
- Singh, U.; Devaraj, S.; Jialal, I. Vitamin E, Oxidative Stress, and Inflammation. Annu. Rev. Nutr. 2005, 25, 151–174.
- Pierpaoli, E.; Cirioni, O.; Barucca, A.; Orlando, F.; Silvestri, C.; Giacometti, A.; Provinciali, M. Vitamin E supplementation in old mice induces antimicrobial activity and improves the efficacy of daptomycin in an animal model of wounds infected with methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2011, 66, 2184–2185.
- Raederstorff, D.; Wyss, A.; Calder, P.; Weber, P.; Eggersdorfer, M. Vitamin E function and requirements in relation to PUFA. Br. J. Nutr. 2015, 114, 1113–1122.
- Lebold, K.M.; Traber, M.G. Interactions between α-tocopherol, polyunsaturated fatty acids, and lipoxygenases during embryogenesis. Free Radic. Biol. Med. 2014, 66, 13–19.
- Hartmann, M.S.; Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Vitamin E as promising adjunct treatment option in the combat of infectious diseases caused by bacterial including multi-drug resistant pathogens—Results from a comprehensive literature survey. Eur. J. Microbiol. Immunol. 2020, 10, 193–201.
- Vergalito, F.; Pietrangelo, L.; Petronio, G.P.; Colitto, F.; Cutuli, M.A.; Magnifico, I.; Venditti, N.; Guerra, G.; Di Marco, R. Vitamin E for prevention of biofilm-caused Healthcare-associated infections. Open Med. 2019, 15, 14–21.
- Mclaughlin, P.; Weihrauch, J.L. Vitamin E content of foods. J. Am. Diet. Assoc. 1979, 75, 647–665.
- Sheppard, A.J.; Pennington, J.A.T.; Weihrauch, J.L. Analysis and distribution of vitamin E in vegetable oils and foods. In Vitamin E in Health and Disease; Packer, L., Fuchs, J., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1993; pp. 7–65. ISBN 9780824786922.
- Chun, J.; Lee, J.; Ye, L.; Exler, J.; Eitenmiller, R.R. Tocopherol and tocotrienol contents of raw and processed fruits and vegetables in the United States diet. J. Food Compos. Anal. 2006, 19, 196–204.
- Brigelius-Flohé, R.; Traber, M.G. Vitamin E: Function and metabolism. FASEB J. 1999, 13, 1145–1155.
- Reboul, E.; Richelle, M.; Perrot, E.; Desmoulins-Malezet, C.; Pirisi, V.; Borel, P. Bioaccessibility of Carotenoids and Vitamin E from Their Main Dietary Sources. J. Agric. Food Chem. 2006, 54, 8749–8755.
- Bjelakovic, G.; Nikolova, D.; Gluud, C. Meta-Regression Analyses, Meta-Analyses, and Trial Sequential Analyses of the Effects of Supplementation with Beta-Carotene, Vitamin A, and Vitamin E Singly or in Different Combinations on All-Cause Mortality: Do We Have Evidence for Lack of Harm? PLoS ONE 2013, 8, e74558.
- Sidgwick, G.P.; McGeorge, D.; Bayat, A. A comprehensive evidence-based review on the role of topicals and dressings in the management of skin scarring. Arch. Dermatol. Res. 2015, 307, 461–477.
- Kuriyama, K.; Shimizu, T.; Horiguchi, T.; Watabe, M.; Abe, Y. Vitamin E ointment at high dose levels suppresses contact dermatitis in rats by stabilizing keratinocytes. Inflamm. Res. 2002, 51, 483–489.
- Niki, E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radic. Biol. Med. 2014, 66, 3–12.
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013.
- Pekmezci, D. Vitamin E and Immunity. Vitam. Horm. 2011, 86, 179–215.
- Szymańska, R.; Nowicka, B.; Kruk, J. Vitamin E—Occurrence, Biosynthesis by Plants and Functions in Human Nutrition. Mini Rev. Med. Chem. 2017, 17, 1039–1052.
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614.
- Schneider, C. Chemistry and biology of vitamin E. Mol. Nutr. Food Res. 2005, 49, 7–30.
- Mocchegiani, E.; Costarelli, L.; Giacconi, R.; Malavolta, M.; Basso, A.; Piacenza, F.; Ostan, R.; Cevenini, E.; Gonos, E.S.; Franceschi, C.; et al. Vitamin E–gene interactions in aging and inflammatory age-related diseases: Implications for treatment. A systematic review. Ageing Res. Rev. 2014, 14, 81–101.
- Naguib, M.M.; Valvano, M.A. Vitamin E Increases Antimicrobial Sensitivity by Inhibiting Bacterial Lipocalin Antibiotic Binding. mSphere 2018, 3, e00564-18.
- Yang, C.; Wu, T.; Qi, Y.; Zhang, Z. Recent Advances in the Application of Vitamin E TPGS for Drug Delivery. Theranostics 2018, 8, 464–485.
- Neophytou, C.M.; Constantinou, C.; Papageorgis, P.; Constantinou, A.I. D-alpha-tocopheryl polyethylene glycol succinate (TPGS) induces cell cycle arrest and apoptosis selectively in Surviving-overexpressing breast cancer cells. Biochem. Pharmacol. 2014, 89, 31–42.
- Guo, Y.; Luo, J.; Tan, S.; Otieno, B.O.; Zhang, Z. The applications of Vitamin E TPGS in drug delivery. Eur. J. Pharm. Sci. 2013, 49, 175–186.
- Jacquemin, E.; Hermeziu, B.; Kibleur, Y.; Friteau, I.; Mathieu, D.; Le Coz, F.; Moyse, D.; Gérardin, M.; Jacqz-Aigrain, E.; Munck, A. Bioavailability of oral vitamin E formulations in adult volunteers and children with chronic cholestasis or cystic fibrosis. J. Clin. Pharm. Ther. 2009, 34, 515–522.
- Prasad, Y.; Puthli, S.; Eaimtrakarn, S.; Ishida, M.; Yoshikawa, Y.; Shibata, N.; Takada, K. Enhanced intestinal absorption of vancomycin with Labrasol and d-α-tocopheryl PEG 1000 succinate in rats. Int. J. Pharm. 2003, 250, 181–190.
- Bogman, K.; Zysset, Y.; Degen, L.; Hopfgartner, G.; Gutmann, H.; Alsenz, J.; Drewe, J. P-glycoprotein and surfactants: Effect on intestinal talinolol absorption. Clin. Pharmacol. Ther. 2005, 77, 24–32.
- Kang, X.-Q.; Shu, G.-F.; Jiang, S.-P.; Xu, X.-L.; Qi, J.; Jin, F.-Y.; Liu, D.; Xiao, Y.-H.; Lu, X.-Y.; Du, Y.-Z. Effective targeted therapy for drug-resistant infection by ICAM-1 antibody-conjugated TPGS modified β-Ga2O3:Cr3+ nanoparticles. Theranostics 2019, 9, 2739–2753.
- Provinciali, M.; Cirioni, O.; Orlando, F.; Pierpaoli, E.; Barucca, A.; Silvestri, C.; Ghiselli, R.; Scalise, A.; Brescini, L.; Guerrieri, M.; et al. Vitamin E improves the in vivo efficacy of tigecycline and daptomycin in an animal model of wounds infected with methicillin-resistant Staphylococcus aureus. J. Med. Microbiol. 2011, 60, 1806–1812.
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006, 78, 2088–2098.
- Suzuki, Y.J.; Tsuchiya, M.; Wassall, S.R.; Choo, Y.M.; Govil, G.; Kagan, V.E.; Packer, L. Structural and dynamic membrane properties of alpha-tocopherol and alpha-tocotrienol: Implication to the molecular mechanism of their antioxidant potency. Biochemistry 1993, 32, 10692–10699.
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tocotrienols: A review. Nutr. Metab. 2014, 11, 52.
- Shahidi, F.; De Camargo, A.C. Tocopherols and Tocotrienols in Common and Emerging Dietary Sources: Occurrence, Applications, and Health Benefits. Int. J. Mol. Sci. 2016, 17, 1745.
- Pearce, B.C.; Parker, R.A.; Deason, M.E.; Dischino, D.D.; Gillespie, E.; Qureshi, A.A.; Wright, J.J.K.; Volk, K. Inhibitors of Cholesterol Biosynthesis. 2. Hypocholesterolemic and Antioxidant Activities of Benzopyran and Tetrahydronaphthalene Analogs of the Tocotrienols. J. Med. Chem. 1994, 37, 526–541.
- Pearce, B.C.; Parker, R.A.; Deason, M.E.; Qureshi, A.A.; Wright, J.J.K. Hypocholesterolemic activity of synthetic and natural tocotrienols. J. Med. Chem. 1992, 35, 3595–3606.
- Pierpaoli, E.; Orlando, F.; Cirioni, O.; Simonetti, O.; Giacometti, A.; Provinciali, M. Supplementation with tocotrienols from Bixa orellana improves the in vivo efficacy of daptomycin against methicillin-resistant Staphylococcus aureus in a mouse model of infected wound. Phytomed. Int. J. Phytother. Phytopharm. 2017, 36, 50–53.
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and Tocotrienols—Bioactive Dietary Compounds; What Is Certain, What Is Doubt? Int. J. Mol. Sci. 2021, 22, 6222.
- Lin, T.S.; Latiff, A.A.; Hamid, N.A.A.; Ngah, W.Z.B.W.; Mazlan, M. Evaluation of Topical Tocopherol Cream on Cutaneous Wound Healing in Streptozotocin-Induced Diabetic Rats. Evid. Based Complement. Altern. Med. 2012, 2012, 491027.
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Ghorbani, S.; Ai, J.; Sahrapeyma, H. Chitosan/alginate hydrogels containing Alpha-tocopherol for wound healing in rat model. J. Drug Deliv. Sci. Technol. 2019, 51, 204–213.
- Bonferoni, M.C.; Sandri, G.; Rossi, S.; Dellera, E.; Invernizzi, A.; Boselli, C.; Cornaglia, A.I.; Del Fante, C.; Perotti, C.; Vigani, B.; et al. Association of Alpha Tocopherol and Ag Sulfadiazine Chitosan Oleate Nanocarriers in Bioactive Dressings Supporting Platelet Lysate Application to Skin Wounds. Mar. Drugs 2018, 16, 56.
- Nurlaily, A.; Azian, A.; Musalmah, M. Tocotrienol-rich fraction formulation enhances wound healing in streptozotocin-induced diabetic rats. Med. Health 2011, 6, 234.
- Elsy, B.; Khan, A.A.; Maheshwari, V. Effect of vitamin E isoforms on the primary intention skin wound healing of diabetic rats. Our Dermatol. Online 2017, 8, 369–375.
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32.
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692.
- Thattaruparambil Raveendran, N.; Mohandas, A.; Ramachandran Menon, R.; Somasekharan Menon, A.; Biswas, R.; Jayakumar, R. Ciprofloxacin- and Fluconazole-Containing Fibrin-Nanoparticle-Incorporated Chitosan Bandages for the Treatment of Polymicrobial Wound Infections. ACS Appl. Bio Mater. 2019, 2, 243–254.
- Marangon, C.A.; Martins, V.; Ling, M.H.; Melo, C.C.; Plepis, A.; Meyer, R.L.; Nitschke, M. Combination of Rhamnolipid and Chitosan in Nanoparticles Boosts Their Antimicrobial Efficacy. ACS Appl. Mater. Interfaces 2020, 12, 5488–5499.
- Baxter, R.M.; Dai, T.; Kimball, J.; Wang, E.; Hamblin, M.R.; Wiesmann, W.P.; McCarthy, S.J.; Baker, S.M. Chitosan dressing promotes healing in third degree burns in mice: Gene expression analysis shows biphasic effects for rapid tissue regeneration and decreased fibrotic signaling. J. Biomed. Mater. Res. Part A 2013, 101, 340–348.
- Shivakumar, P.; Gupta, M.S.; Jayakumar, R.; Gowda, D.V. Prospection of chitosan and its derivatives in wound healing: Proof of patent analysis (2010–2020). Int. J. Biol. Macromol. 2021, 184, 701–712.
- Jhaveri, J.; Raichura, Z.; Khan, T.; Momin, M.; Omri, A. Chitosan Nanoparticles-Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics. Molecules 2021, 26, 272.
- Stanescu, P.-O.; Radu, I.-C.; Alexa, R.L.; Hudita, A.; Tanasa, E.; Ghitman, J.; Stoian, O.; Tsatsakis, A.; Ginghina, O.; Zaharia, C.; et al. Novel chitosan and bacterial cellulose biocomposites tailored with polymeric nanoparticles for modern wound dressing development. Drug Deliv. 2021, 28, 1932–1950.
- Zhang, Y.-J.; Gao, B.; Liu, X.-W. Topical and effective hemostatic medicines in the battlefield. Int. J. Clin. Exp. Med. 2015, 8, 10–19.
- Moghadas, B.; Solouk, A.; Sadeghi, D. Development of chitosan membrane using non-toxic crosslinkers for potential wound dressing applications. Polym. Bull. 2020, 78, 4919–4929.
- Munir, M.U.; Ahmed, A.; Usman, M.; Salman, S. Recent Advances in Nanotechnology-Aided Materials in Combating Microbial Resistance and Functioning as Antibiotics Substitutes. Int. J. Nanomed. 2020, 15, 7329–7358.
- Dai, T.; Tegos, G.P.; Burkatovskaya, M.; Castano, A.P.; Hamblin, M.R. Chitosan Acetate Bandage as a Topical Antimicrobial Dressing for Infected Burns. Antimicrob. Agents Chemother. 2009, 53, 393–400.
- Anjum, S.; Arora, A.; Alam, M.; Gupta, B. Development of antimicrobial and scar preventive chitosan hydrogel wound dressings. Int. J. Pharm. 2016, 508, 92–101.
- Baldrick, P. The safety of chitosan as a pharmaceutical excipient. Regul. Toxicol. Pharmacol. 2010, 56, 290–299.
- Norowski, P.A., Jr.; Fujiwara, T.; Clem, W.C.; Adatrow, P.C.; Eckstein, E.C.; Haggard, W.O.; Bumgardner, J.D. Novel naturally crosslinked electrospun nanofibrous chitosan mats for guided bone regeneration membranes: Material characterization and cytocompatibility. J. Tissue Eng. Regen. Med. 2015, 9, 577–583.
- Oluwole, D.O.; Prinsloo, E.; Nyokong, T. Photophysical behavior and photodynamic therapy activity of conjugates of zinc monocarboxyphenoxy phthalocyanine with human serum albumin and chitosan. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 292–300.
- Senevirathne, M.; Ahn, C.-B.; Kim, S.-K.; Je, J.-Y. Cosmeceutical Applications of Chitosan and Its Derivatives. In Marine Cosmeceuticals: Trends and Prospects; Kim, S.-K., Ed.; CRC Press: Boca Raton, FL, USA, 2011; p. 169.
- Jimtaisong, A.; Saewan, N. Utilization of carboxymethyl chitosan in cosmetics. Int. J. Cosmet. Sci. 2014, 36, 12–21.
- Reynolds, T.; Dweck, A. Aloe vera leaf gel: A review update. J. Ethnopharmacol. 1999, 68, 3–37.
- Boudreau, M.D.; Beland, F.A. An Evaluation of the Biological and Toxicological Properties of Aloe Barbadensis (Miller), Aloe vera. J. Environ. Sci. Health Part C 2006, 24, 103–154.
- Mosayebi, G.; Ghazavi, A.; Aghili, B.; Mirshafiei, A. Immunomodulating Activity of Aloe vera in Animal Model of Multiple Sclerosis. J. Arak Uni. Med. Sci. 2009, 12, 109–115. Available online: http://jams.arakmu.ac.ir/article-1-388-en.html (accessed on 12 December 2021).
- Hekmatpou, D.; Mehrabi, F.; Rahzani, K.; Aminiyan, A. The effect of Aloe vera gel on prevention of pressure ulcers in patients hospitalized in the orthopedic wards: A randomized triple-blind clinical trial. BMC Complement. Altern. Med. 2018, 18, 264.
- West, D.; Zhu, Y.F. Evaluation of Aloe vera gel gloves in the treatment of dry skin associated with occupational exposure. Am. J. Infect. Control 2003, 31, 40–42.
- Eshun, K.; He, Q. Aloe vera: A Valuable Ingredient for the Food, Pharmaceutical and Cosmetic Industries—A Review. Crit. Rev. Food Sci. Nutr. 2004, 44, 91–96.
- Abakar, H.O.M.; Bakhiet, S.E.A.; Abadi, R.S.M. Antimicrobial Activity and Minimum Inhibitory Concentration of Aloe vera Sap and Leaves Using Different Extracts. J. Pharmacogn. Phytochem. 2017, 6, 298–303. Available online: https://www.phytojournal.com/archives/2017/vol6issue3/PartF/6-3-14-403.pdf (accessed on 3 March 2022).
- Goudarzi, M.; Fazeli, M.; Azad, M.; Seyedjavadi, S.S.; Mousavi, R. Aloe vera Gel: Effective Therapeutic Agent against Multidrug-Resistant Pseudomonas aeruginosa Isolates Recovered from Burn Wound Infections. Chemother. Res. Pract. 2015, 2015, 639806.
- Hajhashemi, V.; Ghannadi, A.; Heidari, A. Anti-inflammatory and wound healing activities of Aloe littoralis in rats. Res. Pharm. Sci. 2012, 7, 73–78.
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203.
- Yuan, X.; Han, L.; Fu, P.; Zeng, H.; Lv, C.; Chang, W.; Runyon, R.S.; Ishii, M.; Han, L.; Liu, K.; et al. Cinnamaldehyde accelerates wound healing by promoting angiogenesis via up-regulation of PI3K and MAPK signaling pathways. Lab. Investig. 2018, 98, 783–798.
- Qu, S.; Yang, K.; Chen, L.; Liu, M.; Geng, Q.; He, X.; Li, Y.; Liu, Y.; Tian, J. Cinnamaldehyde, a Promising Natural Preservative against Aspergillus flavus. Front. Microbiol. 2019, 10, 2895.
- Chen, L.; Wang, Z.; Liu, L.; Qu, S.; Mao, Y.; Peng, X.; Li, Y.-X.; Tian, J. Cinnamaldehyde inhibits Candida albicans growth by causing apoptosis and its treatment on vulvovaginal candidiasis and oropharyngeal candidiasis. Appl. Microbiol. Biotechnol. 2019, 103, 9037–9055.
- Fahlbusch, K.-G.; Hammerschmidt, F.-J.; Panten, J.; Pickenhagen, W.; Schatkowski, D.; Bauer, K.; Garbe, D.; Surburg, H. Flavors and Fragrances. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2003; ISBN 978-3-527-30673-2.
- Ma, W.-B.; Feng, J.-T.; Jiang, Z.-L.; Zhang, X. Fumigant Activity of 6 Selected Essential Oil Compounds and Combined Effect of Methyl Salicylate and Trans-Cinnamaldehyde against Culex pipiens pallens. J. Am. Mosq. Control Assoc. 2014, 30, 199–203.
- Ramasamy, M.; Lee, J.-H.; Lee, J. Development of gold nanoparticles coated with silica containing the antibiofilm drug cinnamaldehyde and their effects on pathogenic bacteria. Int. J. Nanomed. 2017, 12, 2813–2828.
- Utchariyakiat, I.; Surassmo, S.; Jaturanpinyo, M.; Khuntayaporn, P.; Chomnawang, M.T. Efficacy of cinnamon bark oil and cinnamaldehyde on anti-multidrug resistant Pseudomonas aeruginosa and the synergistic effects in combination with other antimicrobial agents. BMC Complement. Altern. Med. 2016, 16, 158.
- Pereira, W.; Pereira, C.; Assunção, R.; da Silva, I.; Rego, F.; Alves, L.; Santos, J.; Nogueira, F.; Zagmignan, A.; Thomsen, T.; et al. New Insights into the Antimicrobial Action of Cinnamaldehyde towards Escherichia coli and Its Effects on Intestinal Colonization of Mice. Biomolecules 2021, 11, 302.
- Lee, S.-C.; Wang, S.-Y.; Li, C.-C.; Liu, C.-T. Anti-inflammatory effect of cinnamaldehyde and linalool from the leaf essential oil of Cinnamomum osmophloeum Kanehira in endotoxin-induced mice. J. Food Drug Anal. 2018, 26, 211–220.
- Takasao, N.; Tsuji-Naito, K.; Ishikura, S.; Tamura, A.; Akagawa, M. Cinnamon Extract Promotes Type I Collagen Biosynthesis via Activation of IGF-I Signaling in Human Dermal Fibroblasts. J. Agric. Food Chem. 2012, 60, 1193–1200.
- Ferro, T.A.; Souza, E.B.; Suarez, M.A.; Rodrigues, J.F.; Pereira, D.M.; Mendes, S.J.; Gonzaga, L.F.; Machado, M.C.; Bomfim, M.R.; Calixto, J.B.; et al. Topical Application of Cinnamaldehyde Promotes Faster Healing of Skin Wounds Infected with Pseudomonas aeruginosa. Molecules 2019, 24, 1627.
This entry is offline, you can click here to edit this entry!
