Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Supercapacitors (SCs) have attracted attention as an important energy source for various applications owing to their high power outputs and outstanding energy densities. The electrochemical performance of an SC device is predominantly determined by electrode materials, and thus, the selection and synthesis of the materials are crucial.
- energy storage devices
- supercapacitors
- metal oxides
1. Introduction
The requirement of using energy-storage devices for multifunctional applications is currently a hot research topic. Technological advancements ranging from electric vehicles to miniature portable electronics have considerably increased the demand for energy storage devices. Electrochemical energy storage is the most effective, cheap, and convenient method for meeting this demand [1][2]. Owing to their low costs and versatile performance, batteries and electrochemical capacitors (ECs) are two important categories of electrochemical energy-storage devices [3]. Although batteries are well known for exhibiting high energy densities, their lifespans are short and they exhibit low power densities [4][5][6][7]. In contrast, supercapacitors (SCs) are the most promising electrochemical energy storage devices that bridge the gap between conventional capacitors and batteries. SCs are notable for their unique high power densities, wide working-temperature ranges, extended cycling lives, improved energy densities compared with traditional capacitors, low maintenance, low leakage currents, long service lives, and high efficiencies [8][9][10]. Hence, SCs are employed in a wide range of applications, such as portable electronics, regenerative power-braking systems, hybrid electric vehicles, spacecrafts, aircrafts, military devices, biomedical equipment, and wind-energy power generators [11][12]. As a result, SCs have attracted considerable attention from both research and technological perspectives. However, SCs exhibit low energy densities, which are major drawbacks that limit their use in applications requiring both high energy and power densities. SCs have been broadly categorized as electric double-layer capacitors (EDLCs) and pseudocapacitors (PCs) according to their charge storage mechanisms, as shown in Figure 1 [13].
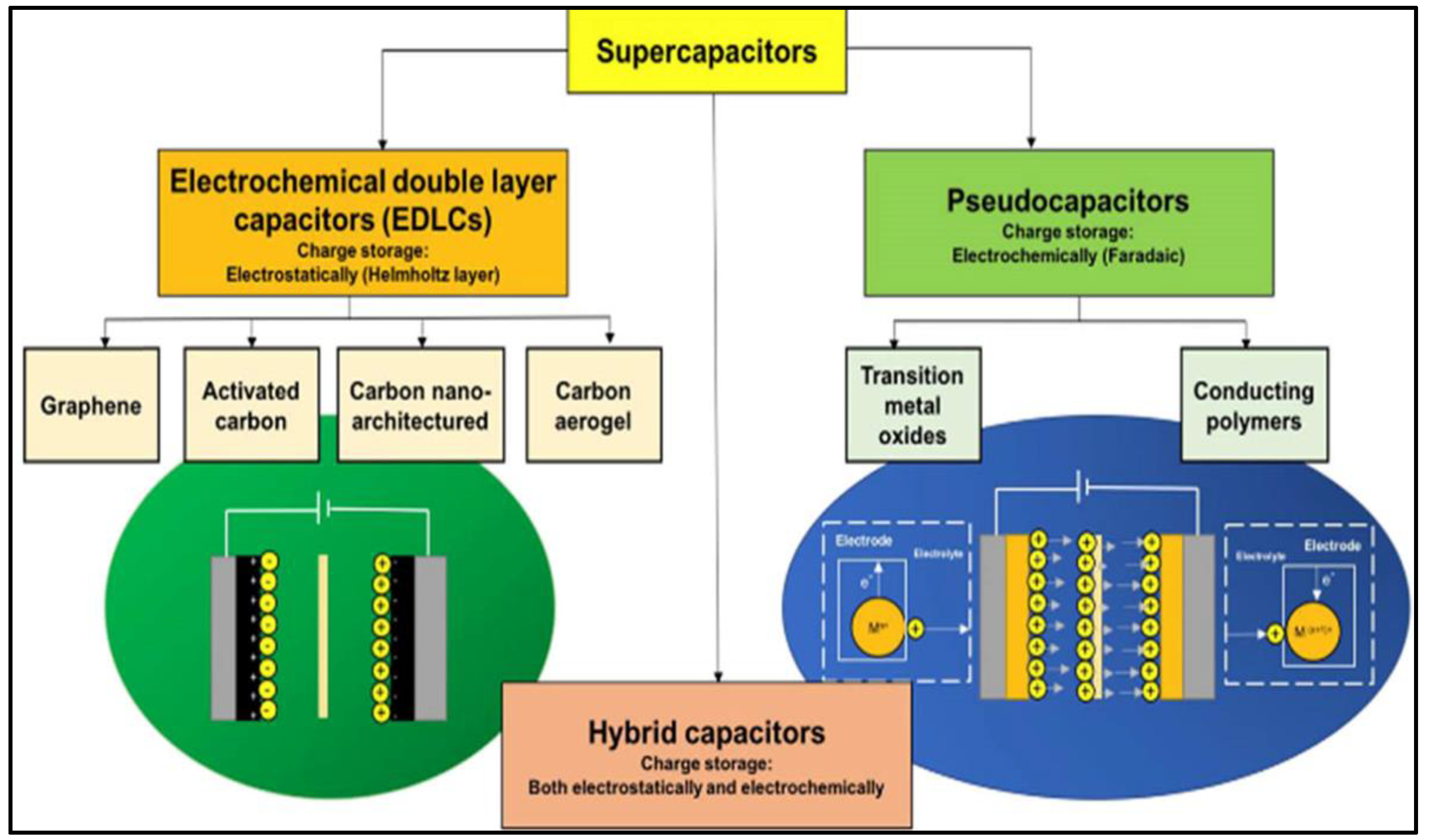
Figure 1. Schematic classifying different SC types and their underlying charge-storage mechanism. Reprinted from Ref. [13].
EDLCs are so named because they physically store an electrostatic charge in an electric field between an adjacent electrode and electrolyte, and the charge rapidly accumulates through electrostatic attraction without any chemical reactions, which generates a high device-power density [13][14][15]. In contrast, PCs operate by faradaically storing a charge at the electrode–electrolyte interface, where the charge is actually transferred between the electrode and the electrolyte. Through reversible redox reactions involving the highly reversible formation of chemical species, PCs exhibit higher energy densities than EDLCs [16][17][18]. The main components required to fabricate a hybrid-operating SC device include electrode materials, electrolytes, separators, and current collectors [19]. Although each component is important, selecting an electrode’s material and fabricating the electrode are the most crucial aspects. Carbon-based materials are the best EDLC electrode materials because their charge-storage mechanism mainly relies on the electrode-material surface area accessible to electrolyte ions. Activated carbon (AC), graphene and its derivatives, carbon nanotubes (CNTs), and carbon aerogels have been used as EDLC electrode materials [20][21][22] because they are inexpensive, are simply fabricated, and exhibit large surface areas and wide ranges of pore-size distributions. However, owing to their low specific capacitances, carbonaceous materials do not sufficiently contribute to the high SC energy density [23]. High-capacitance electrode materials are preferentially used to overcome the low energy density. Owing to their rapid reversible surface-redox reactions, conducting polymers and metal oxides (MOs) are pseudocapacitive electrode materials that provide high energy densities to SCs [24][25][26]. Compared to MOs, conducting polymers exhibit poor structural stabilities during continuous charge–discharge cycling. Therefore, various low-cost MOs and their composite electrode materials have been identified as promising alternatives to carbonaceous materials and conducting polymers [27][28][29]. Because of their exceptional physicochemical properties, MOs have been used in several applications (Scheme 1) and are usually applied as electrode materials because they can be synthesized using a wide range of methods and because of their high theoretical specific capacitances, natural abundance, excellent pseudocapacitive behaviors, high thermal and electrochemical stabilities, and reliabilities [30][31][32][33].
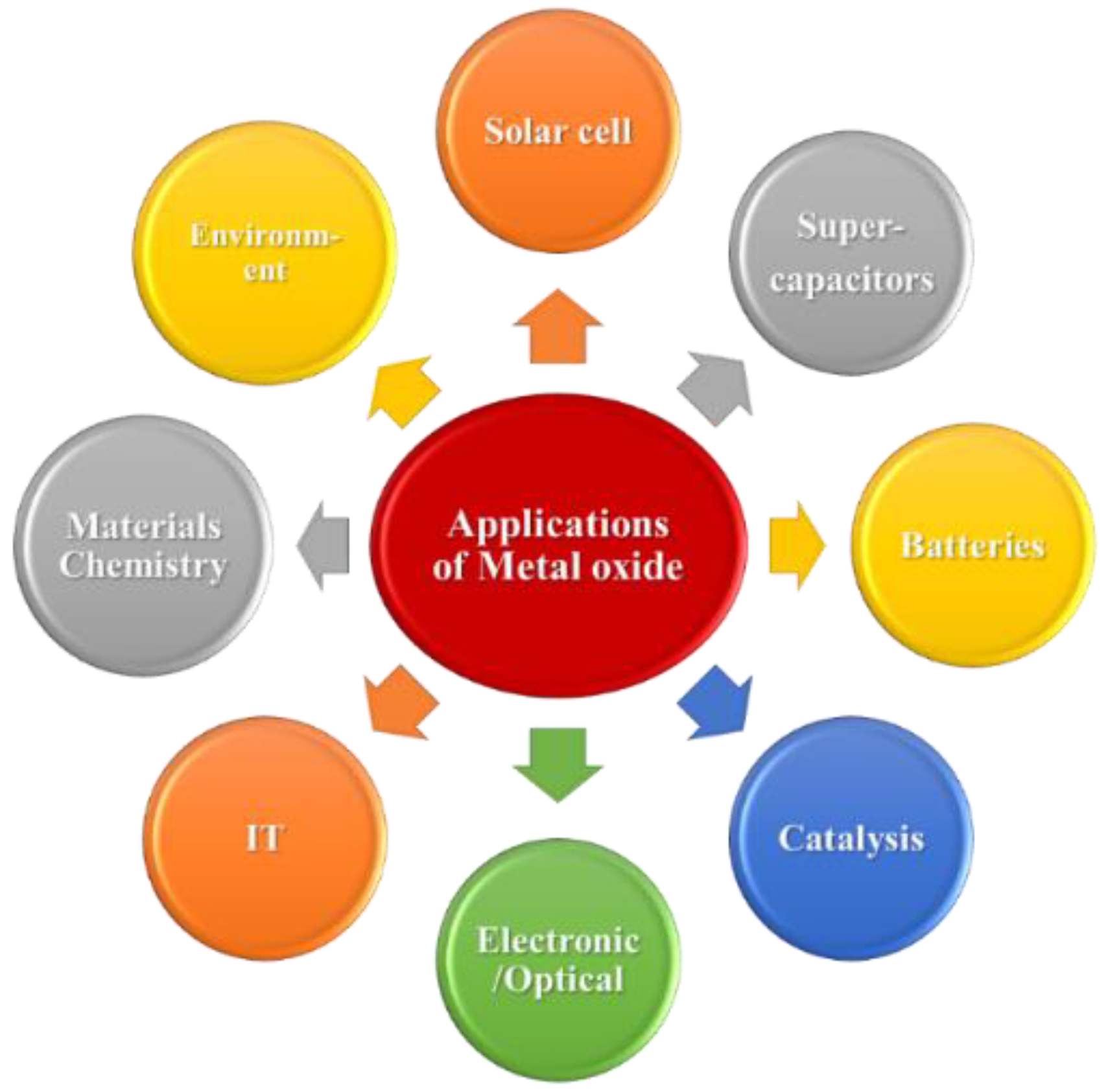
Scheme 1. Different MO material applications.
MO-based SC electrodes have been synthesized using various chemical and vapor-phase-based techniques, including hydrothermal synthesis [34], sol-gel processing [35], electrochemical/electropolymerization synthesis [36], chemical bath deposition (CBD) [37], solvothermal synthesis [38], microwave-assisted synthesis [39], atomic-layer deposition (ALD) [40], and ball milling (Scheme 2). However, not[41] all these techniques can be applied to industrially produce high-quality low-cost MO-based SC electrodes. Therefore, an easy, efficient, economic, nontoxic, and reliable technique that can be used to produce numerous high-purity materials under mild reaction conditions should be developed for fabricating MO-based SC electrodes [42][43][44][45].
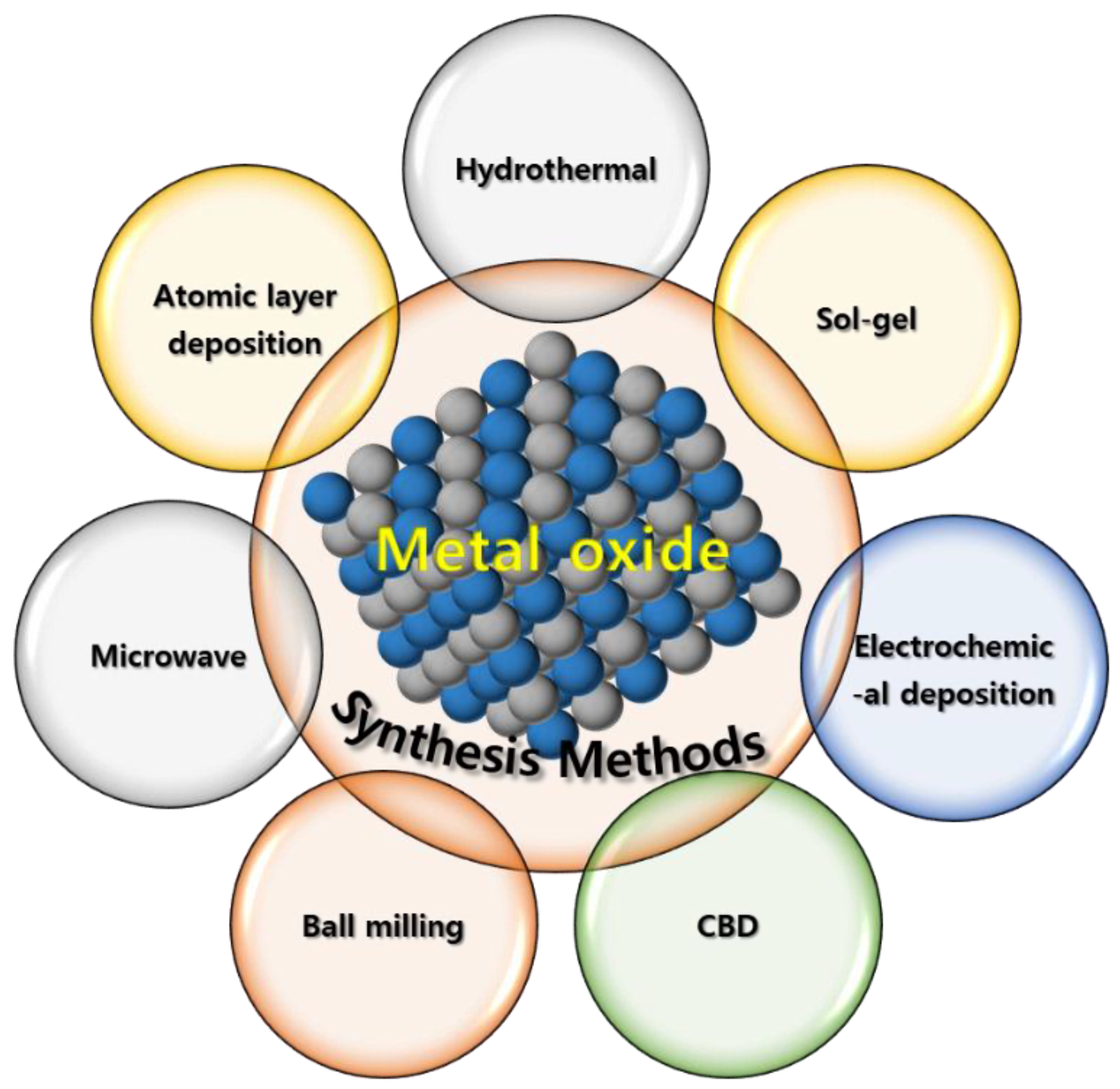
Scheme 2. Different techniques for preparing MO materials..
2. Hydrothermal Synthesis
Hydrothermal synthesis is the most widely used solution-based technique for fabricating SC electrodes; it enables electrode materials exhibiting one-, two-, and three-dimensional and hierarchical nanoarchitectures and well-defined morphologies to be fabricated [46]. Moreover, hydrothermal synthesis facilitates chemical reactions in aqueous media at desired pressures and temperatures [47][48]; chemicals are synthesized above the water boiling point, and water serves as an aqueous medium. With an increasing temperature, pressure begins to increase inside a sealed chamber and consequently initiates a thermochemical reaction. Materials are hydrothermally synthesized in a TeflonTM-lined stainless-steel autoclave [49], which is then placed in an oven and maintained at appropriate temperatures. Hydrothermal synthesis is simple and often referred to as a “environmentally friendly” because it promotes a low chemical consumption and does not emit any toxic gases. Moreover, hydrothermal synthesis is inexpensive, simple to use, requires few experimental conditions, and helps generate a wide range of materials for electrodes exhibiting well-defined morphologies [50][51][52][53][54][55].
3. Microwave-Assisted Synthesis
Microwave-assisted synthesis is a novel and appropriate technique for synthesizing the inorganic materials used in various industrial applications and is a rapidly growing research area. Microwaves have a wide range of applications, including food preparation, mining, rubber and plastic treatment, and wood drying [56][57][58]. Microwave-assisted synthesis can be used to rapidly, easily, inexpensively, uniformly, and efficiently heat materials. Compared to the conventional heating methods used to synthesize porous materials, organic and inorganic compounds, and nanocrystalline materials, microwave-assisted synthesis is advantageous for preparing MOs and fabricating their composite-based SC electrodes because it produces various multidimensional morphologies and crystal structures [59][60][61]. In conventional material heating methods, thermal energy is transferred convectively to a material’s surface and conductively to the material’s bulk. Microwaves are nonionizing electromagnetic radiation exhibiting wavelengths between those of radio and infrared waves and frequencies ranging from 0.3 to 300 MHz. Microwaves convert electromagnetic energy into thermal energy [62]. An electromagnetic field heats material through molecular interactions, and microwaves penetrate the material to provide energy, which entails dielectric heating through a dipole rotation and resonance energy absorption. Because the heat is molecularly transferred, the material is uniformly heated throughout its volume. Therefore, when microwave energy interacts with different dielectric materials, it selectively couples with higher-loss-tangent materials. A microwave heating instrument comprises six main components that generate microwaves.
This entry is adapted from the peer-reviewed paper 10.3390/nano12111873
References
- Parveen, N.; Ansari, S.A.; Ansari, M.Z.; Ansari, M.O. Manganese oxide as an effective electrode material for energy storage: A review. Environ. Chem. Lett. 2021, 20, 283–309.
- Braff, W.A.; Mueller, J.M.; Trancik, J.E. Value of storage technologies for wind and solar energy. Nat. Clim. Chang. 2016, 6, 964–969.
- El-Kady, M.F.; Shao, Y.; Kaner, R.B. Graphene for batteries, supercapacitors and beyond. Nat. Rev. Mater. 2016, 1, 16033.
- Larcher, D.; Tarascon, J.-M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2014, 7, 19–29.
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013.
- Zhang, C.; Wei, Y.-L.; Cao, P.-F.; Lin, M.-C. Energy storage system: Current studies on batteries and power condition system. Renew. Sustain. Energy Rev. 2018, 82, 3091–3106.
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964.
- Hung, K.; Masarapu, C.; Ko, T.; Wei, B. Wide-temperature range operation supercapacitors from nanostructured activated carbon fabric. J. Power Sources 2009, 193, 944–949.
- Zhao, J.; Burke, A.F. Electrochemical capacitors: Materials, technologies and performance. Energy Storage Mater. 2020, 36, 31–55.
- Forse, A.C.; Merlet, C.; Griffin, J.M.; Grey, C.P. New Perspectives on the Charging Mechanisms of Supercapacitors. J. Am. Chem. Soc. 2016, 138, 5731–5744.
- Libich, J.; Máca, J.; Vondrák, J.; Čech, O.; Sedlaříková, M. Supercapacitors: Properties and applications. J. Energy Storage 2018, 17, 224–227.
- Yadav, S.; Devi, A. Recent advancements of metal oxides/Nitrogen-doped graphene nanocomposites for supercapacitor electrode materials. J. Energy Storage 2020, 30, 101486.
- Abdah, M.A.A.M.; Azman, N.H.N.; Kulandaivalu, S.; Sulaiman, Y. Review of the use of transition-metal-oxide and conducting polymer-based fibres for high-performance supercapacitors. Mater. Des. 2020, 186, 108199.
- Wang, T.; Zang, X.; Wang, X.; Gu, X.; Shao, Q.; Cao, N. Recent advances in fluorine-doped/fluorinated carbon-based materials for supercapacitors. Energy Storage Mater. 2020, 30, 367–384.
- Bose, S.; Kuila, T.; Mishra, A.K.; Rajasekar, R.; Kim, N.H.; Lee, J.H. Carbon-based nanostructured materials and their composites as supercapacitor electrodes. J. Mater. Chem. 2011, 22, 767–784.
- Sun, J.; Wu, C.; Sun, X.; Hu, H.; Zhi, C.; Hou, L.; Yuan, C. Recent progresses in high-energy-density all pseudocapacitive-electrode-materials-based asymmetric supercapacitors. J. Mater. Chem. A 2017, 5, 9443–9464.
- Li, R.; Gao, N.; Wang, C.; Ding, G.; Wang, Y.; Ma, H. A facile strategy to in situ synthesize metal oxide/conductive polymer hybrid electrodes for supercapacitors. Soft Matter 2022, 18, 2517–2521.
- De, B.; Banerjee, S.; Verma, K.D.; Pal, T.; Manna, P.K.; Kar, K.K. Transition Metal Oxides as Electrode Materials for Supercapacitors. In Handbook of Nanocomposite Supercapacitor Materials, II; Springer: Cham, Switzerland, 2020; pp. 89–111.
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.-H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473.
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Liu, K.; Jiang, S. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2020, 56, 173–200.
- Ran, F.; Yang, X.; Shao, L. Recent progress in carbon-based nanoarchitectures for advanced supercapacitors. Adv. Compos. Hybrid Mater. 2018, 1, 32–55.
- Borenstein, A.; Hanna, O.; Attias, R.; Luski, S.; Brousse, T.; Aurbach, D. Carbon-based composite materials for supercapacitor electrodes: A review. J. Mater. Chem. A 2017, 5, 12653–12672.
- Wang, J.; Zhang, X.; Li, Z.; Ma, Y.; Ma, L. Recent progress of biomass-derived carbon materials for supercapacitors. J. Power Sources 2020, 451, 227794.
- Ansari, M.Z.; Ansari, S.A.; Parveen, N.; Cho, M.H.; Song, T. Lithium ion storage ability, supercapacitor electrode performance, and photocatalytic performance of tungsten disulfide nanosheets. New J. Chem. 2018, 42, 5859–5867.
- Eliad, L.; Salitra, G.; Soffer, A.; Aurbach, D. Ion sieving effects in the electrical double layer of porous carbon electrodes: Estimating effective ion size in electrolytic solutions. J Phys Chem B 2001, 105, 6880–6887.
- Wang, T.; Chen, H.C.; Yu, F.; Zhao, X.S.; Wang, H. Boosting the cycling stability of transition metal compounds-based supercapacitors. Energy Storage Mater. 2019, 16, 545–573.
- Low, W.H.; Khiew, P.S.; Lim, S.S.; Siong, C.W.; Ezeigwe, E.R. Recent development of mixed transition metal oxide and graphene/mixed transition metal oxide based hybrid nanostructures for advanced supercapacitors. J. Alloys Compd. 2019, 775, 1324–1356.
- Zheng, J.H.; Zhang, R.M.; Yu, P.F.; Wang, X.G. Binary transition metal oxides (BTMO) (Co-Zn, Co-Cu) synthesis and high supercapacitor performance. J. Alloys Compd. 2019, 772, 359–365.
- Zhao, X.; Mao, L.; Cheng, Q.; Li, J.; Liao, F.; Yang, G.; Xie, L.; Zhao, C.; Chen, L. Two-dimensional Spinel Structured Co-based Materials for High Performance Supercapacitors: A Critical Review. Chem. Eng. J. 2020, 387, 124081.
- Delbari, S.A.; Ghadimi, L.S.; Hadi, R.; Farhoudian, S.; Nedaei, M.; Babapoor, A.; Namini, A.S.; Van Le, Q.; Shokouhimehr, M.; Asl, M.S.; et al. Transition metal oxide-based electrode materials for flexible supercapacitors: A review. J. Alloys Compd. 2021, 857, 158281.
- Ansari, A.R.; Ansari, S.A.; Parveen, N.; Ansari, M.O.; Osman, Z. Silver Nanoparticle Decorated on Reduced Graphene Oxide-Wrapped Manganese Oxide Nanorods as Electrode Materials for High-Performance Electrochemical Devices. Crystals 2022, 12, 389.
- Yuan, S.; Duan, X.; Liu, J.; Ye, Y.; Lv, F.; Liu, T.; Wang, Q.; Zhang, X. Recent progress on transition metal oxides as advanced materials for energy conversion and storage. Energy Storage Mater. 2021, 42, 317–369.
- Yang, T.; Song, T.T.; Callsen, M.; Zhou, J.; Chai, J.W.; Feng, Y.P.; Wang, S.J.; Yang, M. Atomically thin 2D transition metal oxides: Structural reconstruction, interaction with substrates, and potential applications. Adv. Mater. Interfaces 2019, 6, 1801160.
- Moyseowicz, A.; Grażyna, G. Hydrothermal-assisted synthesis of a porous polyaniline/reduced graphene oxide composite as a high-performance electrode material for supercapacitors. Compos. Part B Eng. 2019, 159, 4–12.
- Merabet, L.; Kamel, R.; Nawel, B. Sol-gel synthesis, characterization, and supercapacitor applications of MCo2O4 (M= Ni, Mn, Cu, Zn) cobaltite spinels. Ceram. Int. 2018, 44, 11265–11273.
- Youssry, S.M.; El-Hallag, I.S.; Kumar, R.; Kawamura, G.; Matsuda, A.; El-Nahass, M.N. Synthesis of mesoporous Co(OH)2 nanostructure film via electrochemical deposition using lyotropic liquid crystal template as improved electrode materials for supercapacitors application. J. Electroanal. Chem. 2020, 857, 113728.
- Zhao, F.; Huang, W.; Zhou, D. Chemical bath deposition synthesis of nickel cobalt oxides/sulfides for high-performance supercapacitors electrode materials. J. Alloys Compd. 2018, 755, 15–23.
- Yuan, R.; Li, H.; Zhang, X.; Zhu, H.; Zhao, J.; Chen, R. Facile one-pot solvothermal synthesis of bifunctional chrysanthemum-like cobalt-manganese oxides for supercapacitor and degradation of pollutants. J. Energy Storage 2020, 29, 101300.
- Seevakan, K.; Manikandan, A.; Devendran, P.; Shameem, A.; Alagesan, T. Microwave combustion synthesis, magneto-optical and electrochemical properties of NiMoO4 nanoparticles for supercapacitor application. Ceram. Int. 2018, 44, 13879–13887.
- Gandla, D.; Tan, D.Q. Progress Report on Atomic Layer Deposition Toward Hybrid Nanocomposite Electrodes for Next Generation Supercapacitors. Adv. Mater. Interfaces 2019, 6, 1900678.
- Buldu-Akturk, M.; Toufani, M.; Tufani, A.; Erdem, E. ZnO and reduced graphene oxide electrodes for all-in-one supercapacitor devices. Nanoscale 2022, 14, 3269–3278.
- Bathula, C.; Rabani, I.; Ramesh, S.; Lee, S.-H.; Palem, R.R.; Ahmed, A.T.A.; Kim, H.S.; Seo, Y.-S.; Kim, H.-S. Highly efficient solid-state synthesis of Co3O4 on multiwalled carbon nanotubes for supercapacitors. J. Alloys Compd. 2021, 887, 161307.
- Raghavendra, K.V.G.; Vinoth, R.; Zeb, K.; Gopi, C.V.M.; Sambasivam, S.; Kummara, M.R.; Obaidat, I.M.; Kim, H.J. An intuitive review of supercapacitors with recent progress and novel device applications. J. Energy Storage 2020, 31, 101652.
- Lokhande, P.E.; Chavan, U.S.; Pandey, A. Materials and Fabrication Methods for Electrochemical Supercapacitors: Overview. Electrochem. Energy Rev. 2019, 3, 155–186.
- Yumak, T.; Bragg, D.; Sabolsky, E.M. Effect of synthesis methods on the surface and electrochemical characteristics of metal oxide/activated carbon composites for supercapacitor applications. Appl. Surf. Sci. 2019, 469, 983–993.
- Yadav, S.; Sharma, A. Importance and challenges of hydrothermal technique for synthesis of transition metal oxides and composites as supercapacitor electrode materials. J. Energy Storage 2021, 44, 103295.
- Wei, L.; Sevilla, M.; Fuertes, A.B.; Mokaya, R.; Yushin, G. Hydrothermal Carbonization of Abundant Renewable Natural Organic Chemicals for High-Performance Supercapacitor Electrodes. Adv. Energy Mater. 2011, 1, 356–361.
- Kubra, K.T.; Sharif, R.; Patil, B.; Javaid, A.; Shahzadi, S.; Salman, A.; Siddique, S.; Ali, G. Hydrothermal synthesis of neodymium oxide nanoparticles and its nanocomposites with manganese oxide as electrode materials for supercapacitor application. J. Alloys Compd. 2020, 815, 152104.
- Walton, R.I. Subcritical solvothermal synthesis of condensed inorganic materials. Chem. Soc. Rev. 2002, 31, 230–238.
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process. ACS Nano 2010, 4, 4324–4330.
- Wei, H.; Wang, J.; Yang, S.; Zhang, Y.; Li, T.; Zhao, S. Facile hydrothermal synthesis of one-dimensional nanostructured α-MnO2 for supercapacitors. Phys. E Low-Dimens. Syst. Nanostruct. 2016, 83, 41–46.
- Yan, X.; Tong, X.; Wang, J.; Gong, C.; Zhang, M.; Liang, L. Synthesis of mesoporous NiO nanoflake array and its enhanced electrochemical performance for supercapacitor application. J. Alloys Compd. 2014, 593, 184–189.
- Wang, H.; Zhang, L.; Tan, X.; Holt, C.M.; Zahiri, B.; Olsen, B.C.; Mitlin, D. Supercapacitive Properties of Hydrothermally Synthesized Co3O4 Nanostructures. J. Phys. Chem. C 2011, 115, 17599–17605.
- Zhou, E.; Wang, C.; Zhao, Q.; Li, Z.; Shao, M.; Deng, X.; Liu, X.; Xu, X. Facile synthesis of MoO2 nanoparticles as high performance supercapacitor electrodes and photocatalysts. Ceram. Int. 2016, 42, 2198–2203.
- Huang, L.; Zhang, W.; Xiang, J.; Xu, H.; Li, G.; Huang, Y. Hierarchical core-shell NiCo2O4@NiMoO4 nanowires grown on carbon cloth as integrated electrode for high-performance supercapacitors. Sci. Rep. 2016, 6, 31465.
- Schuetz, M.B.; Xiao, L.; Lehnen, T.; Fischer, T.; Mathur, S. Microwave-assisted synthesis of nanocrystalline binary and ternary metal oxides. Int. Mater. Rev. 2017, 63, 341–374.
- Motasemi, F.; Ani, F. A review on microwave-assisted production of biodiesel. Renew. Sustain. Energy Rev. 2012, 16, 4719–4733.
- Das, S.; Mukhopadhyay, A.K.; Datta, S.; Basu, D. Prospects of microwave processing: An overview. Bull. Mater. Sci. 2009, 32, 1–13.
- Wang, Y.; Iqbal, Z.; Mitra, S. Rapid, low temperature microwave synthesis of novel carbon nanotube–silicon carbide composite. Carbon 2006, 44, 2804–2808.
- Zheng, Y.; Tian, Y.; Liu, S.; Tan, X.; Wang, S.; Guo, Q.; Luo, J.; Li, Z. One-step microwave synthesis of NiO/ CNT nanocomposites for high-cycling-stability supercapacitors. J. Alloys Compd. 2019, 806, 170–179.
- Tian, Y.; Du, H.; Zhang, M.; Zheng, Y.; Guo, Q.; Zhang, H.; Luo, J.; Zhang, X. Microwave synthesis of MoS2/MoO2@ CNT nanocomposites with excellent cycling stability for supercapacitor electrodes. J. Mater. Chem. C 2019, 7, 9545–9555.
- Devi, N.; Sahoo, S.; Kumar, R.; Singh, R.K. A review of the microwave-assisted synthesis of carbon nanomaterials, metal oxides/hydroxides and their composites for energy storage applications. Nanoscale 2021, 13, 11679–11711.
This entry is offline, you can click here to edit this entry!
