Transcatheter aortic valve replacement (TAVR) is expanding towards a low-risk patient category as a result of technical advances and operators’ improved skills. However, the post-TAVR antithrombotic regimen remains challenging. Single antiplatelet therapy appears to be the best compromise when there is no compelling indication for chronic oral anticoagulation. Whether it should be aspirin or clopidogrel is not established. There is no supportive evidence to use oral anticoagulation when there is no established indication for oral anticoagulation other than the TAVR procedure. The gap in evidence as to whether DOACs should be preferred over VKA remains when there is an indication for oral anticoagulation (OAC) use. It seems that DOACs are not the same and randomized trials are awaited. Likewise, whether oral anticoagulant therapy should be continued or interrupted during the procedure remains unclear.
- TAVR
- antithrombotic therapy
- oral anticoagulation
- DOAC
- VKA
- SAPT
1. Bleeding Events after TAVR
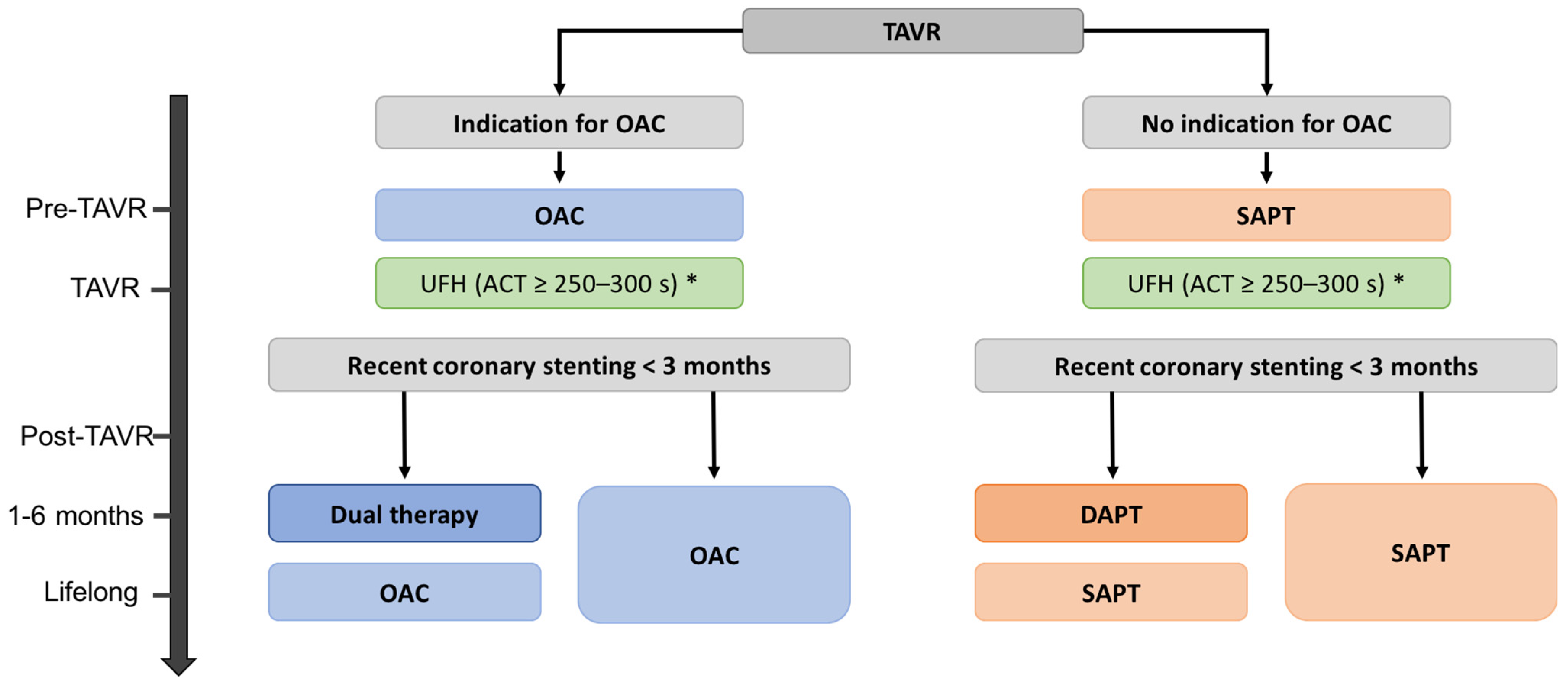
2. Periprocedural Antithrombotic Therapy
3. Antithrombotic Therapy after TAVR
3.1. Antiplatelet Therapy after TAVR: Updated Guidelines in 2021
| Guidelines and Expert Consensus | Recommendations | Class of Recommendation | Level of Evidence |
|---|---|---|---|
| ESC/EACTS 2021 Guidelines [18] | |||
| Patients without underlying indication for chronic OAC | |||
| Lifelong single antiplatelet therapy (aspirin 75–100 mg daily or clopidogrel 75mg daily) is recommended after TAVR in patients with no baseline indication for OAC | I | A | |
| Routine use OAC is not recommended in patients with no baseline indication for OAC | III | B | |
| Patients with underlying indication for chronic OAC | |||
| OAC is recommended lifelong for TAVR patients who have other indications for OAC | I | B | |
| AHA/ACC 2020 Guidelines [24] | |||
| Patients without underlying indication for chronic OAC | |||
| For patients with a bioprosthetic TAVR, aspirin 75–100 mg daily is reasonable in the absence of other indications for oral anticoagulants. | IIa | B-R | |
| For patients with a bioprosthetic TAVR who are at low risk of bleeding, dual antiplatelet therapy with aspirin 75–100 mg and clopidogrel 75 mg may be reasonable for 3–6 months after valve implantation. | IIb | B-NR | |
| For patients with a bioprosthetic TAVR who are at low risk of bleeding, anticoagulation with a VKA to achieve an INR of 2.5 may be reasonable for at least 3 months after valve implantation. | IIb | B-NR | |
| For patients with a bioprosthetic TAVR, treatment with low-dose rivaroxaban (10 mg daily) plus aspirin (75-100 mg) is contraindicated in absence of other indications for oral anticoagulants. | III | B-R | |
| Patients with underlying indication for chronic OAC | |||
| No specific recommendation | |||
| CCS 2019 Position Statement [31] | |||
| Patients without underlying indication for chronic OAC | |||
| Lifelong aspirin 75–100 mg daily | Expert consensus | ||
| In patients with a recent PCI, dual antiplatelet therapy (aspirin 75–100 mg/d plus clopidogrel 75 mg/d) may be continued as per the treating physician | Expert consensus | ||
| Patients with underlying indication for chronic OAC | |||
| DOAC for patients with atrial fibrillation unless contra-indicated* in addition to aspirin for TAVR patients | Expert consensus | ||
| Oral anticoagulation for other indications as per standard guidelines | Expert consensus | ||
| It is prudent to avoid triple therapy in patients at increased risk of bleeding. | Expert consensus | ||
| ACCF/AATS/SCAI/STS 2012 Expert Consensus [20] | |||
| Patients without underlying indication for chronic OAC | |||
| Antiplatelet therapy for at least 3–6 months after TAVR is recommended to decrease the risk of thrombotic or thromboembolic complications | Expert consensus | ||
| Patients with underlying indication for chronic OAC | |||
| In patients treated with warfarin, a direct thrombin inhibitor, or factor Xa inhibitor, it is reasonable to continue low-dose aspirin, but other antiplatelet therapy should be avoided, if possible | Expert consensus | ||
| ACCP-2012 Clinical practice guidelines [32] | |||
| Patients without underlying indication for chronic OAC | |||
| Aspirin (50–100 mg/d) plus clopidogrel (75 mg/d) over VKA therapy and over no platelet therapy in the first 3 months | 2 | C | |
| Patients with underlying indication for chronic OAC | |||
| No specific recommendation | |||
3.2. In Antiplatelet Monotherapy, Use of Aspirin or Anti-P2Y12?
3.3. Patients with Life-Long Indication for Anticoagulation
3.4. In Patients Requiring OAC, Which Anticoagulant to Choose?
3.5. Patients without Underlying Indication for Anticoagulation
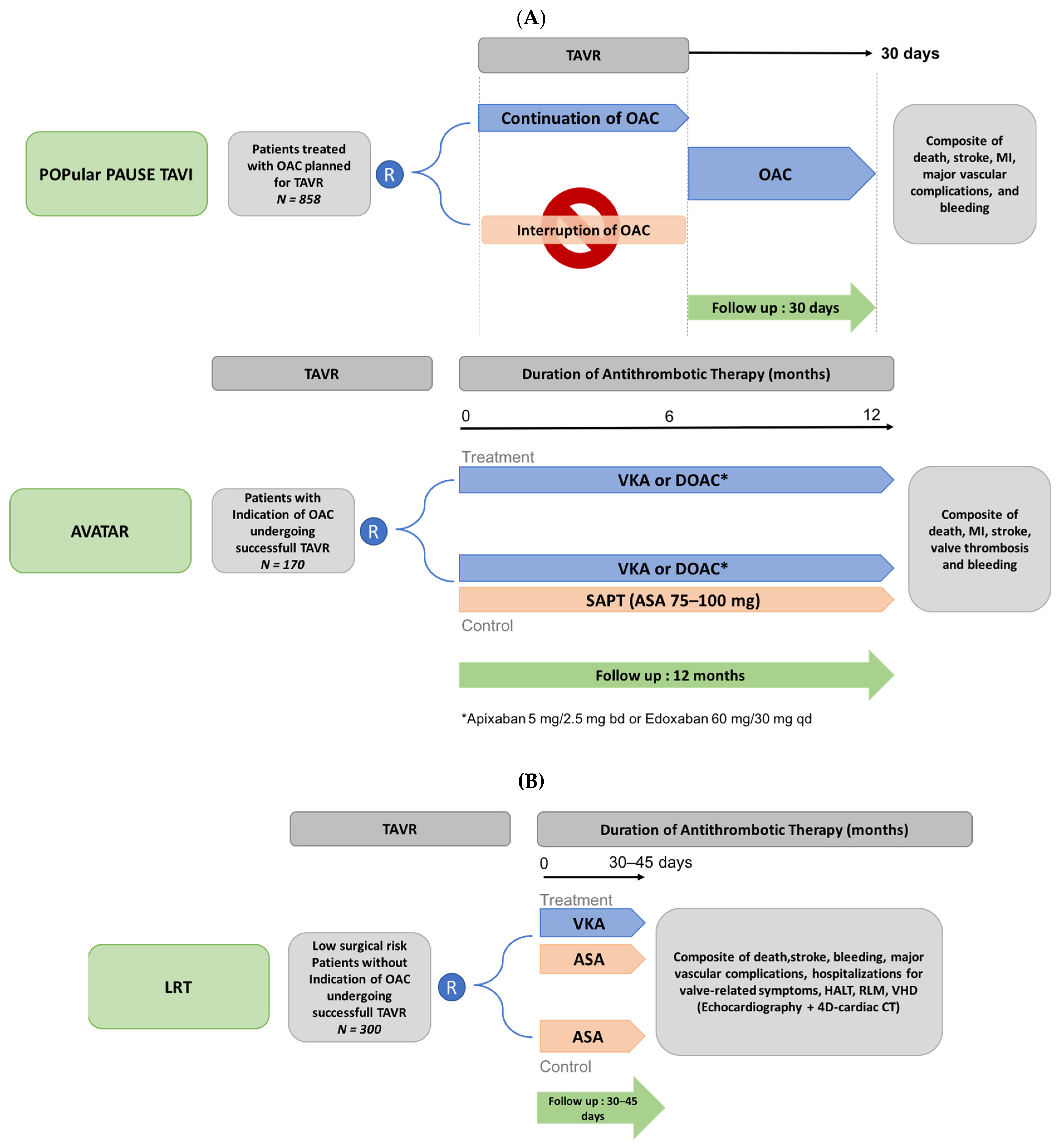
3.6. Ongoing Trials Evaluating Antithrombotic Therapy after TAVR
This entry is adapted from the peer-reviewed paper 10.3390/jcm11082190
References
- Levett, J.Y.; Windle, S.B.; Filion, K.B.; Brunetti, V.C.; Eisenberg, M.J. Meta-Analysis of Transcatheter Versus Surgical Aortic Valve Replacement in Low Surgical Risk Patients. Am. J. Cardiol. 2020, 125, 1230–1238.
- Kappetein, A.P.; Head, S.J.; Généreux, P.; Piazza, N.; van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.-A.; et al. Updated Standardized Endpoint Definitions for Transcatheter Aortic Valve Implantation: The Valve Academic Research Consortium-2 Consensus Document (VARC-2). Eur. J. Cardiothorac. Surg. 2012, 42, S45–S60.
- Varc-3 Writing Committee; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3: Updated Endpoint Definitions for Aortic Valve Clinical Research. Eur. Heart J. 2021, 42, 1825–1857.
- Piccolo, R.; Pilgrim, T.; Franzone, A.; Valgimigli, M.; Haynes, A.; Asami, M.; Lanz, J.; Räber, L.; Praz, F.; Langhammer, B.; et al. Frequency, Timing, and Impact of Access-Site and Non-Access-Site Bleeding on Mortality among Patients Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2017, 10, 1436–1446.
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715.
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705.
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. N. Engl. J. Med. 2010, 363, 1597–1607.
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N. Engl. J. Med. 2011, 364, 2187–2198.
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Prosthesis. N. Engl. J. Med. 2014, 370, 1790–1798.
- Thyregod, H.G.H.; Steinbrüchel, D.A.; Ihlemann, N.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients with Severe Aortic Valve Stenosis: 1-Year Results from the All-Comers NOTION Randomized Clinical Trial. J. Am. Coll. Cardiol. 2015, 65, 2184–2194.
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620.
- Reardon, M.J.; Van Mieghem, N.M.; Popma, J.J.; Kleiman, N.S.; Søndergaard, L.; Mumtaz, M.; Adams, D.H.; Deeb, G.M.; Maini, B.; Gada, H.; et al. Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2017, 376, 1321–1331.
- Généreux, P.; Cohen, D.J.; Mack, M.; Rodes-Cabau, J.; Yadav, M.; Xu, K.; Parvataneni, R.; Hahn, R.; Kodali, S.K.; Webb, J.G.; et al. Incidence, Predictors, and Prognostic Impact of Late Bleeding Complications after Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2014, 64, 2605–2615.
- Wang, J.; Yu, W.; Jin, Q.; Li, Y.; Liu, N.; Hou, X.; Yu, Y. Risk Factors for Post-TAVI Bleeding According to the VARC-2 Bleeding Definition and Effect of the Bleeding on Short-Term Mortality: A Meta-Analysis. Can. J. Cardiol. 2017, 33, 525–534.
- Borz, B.; Durand, E.; Godin, M.; Tron, C.; Canville, A.; Litzler, P.-Y.; Bessou, J.-P.; Cribier, A.; Eltchaninoff, H. Incidence, Predictors and Impact of Bleeding after Transcatheter Aortic Valve Implantation Using the Balloon-Expandable Edwards Prosthesis. Heart 2013, 99, 860–865.
- Kochman, J.; Rymuza, B.; Huczek, Z.; Kołtowski, Ł.; Ścisło, P.; Wilimski, R.; Ścibisz, A.; Stanecka, P.; Filipiak, K.J.; Opolski, G. Incidence, Predictors and Impact of Severe Periprocedural Bleeding According to VARC-2 Criteria on 1-Year Clinical Outcomes in Patients After Transcatheter Aortic Valve Implantation. Int. Heart J. 2016, 57, 35–40.
- Nijenhuis, V.J.; Ten Berg, J.M.; Hengstenberg, C.; Lefèvre, T.; Windecker, S.; Hildick-Smith, D.; Kupatt, C.; Van Belle, E.; Tron, C.; Hink, H.U.; et al. Usefulness of Clopidogrel Loading in Patients Who Underwent Transcatheter Aortic Valve Implantation (from the BRAVO-3 Randomized Trial). Am. J. Cardiol. 2019, 123, 1494–1500.
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2021, 43, 561–632.
- Dangas, G.D.; Lefèvre, T.; Kupatt, C.; Tchetche, D.; Schäfer, U.; Dumonteil, N.; Webb, J.G.; Colombo, A.; Windecker, S.; Ten Berg, J.M.; et al. Bivalirudin Versus Heparin Anticoagulation in Transcatheter Aortic Valve Replacement: The Randomized BRAVO-3 Trial. J. Am. Coll. Cardiol. 2015, 66, 2860–2868.
- Holmes, D.R.; Mack, M.J.; Kaul, S.; Agnihotri, A.; Alexander, K.P.; Bailey, S.R.; Calhoon, J.H.; Carabello, B.A.; Desai, M.Y.; Edwards, F.H.; et al. 2012 ACCF/AATS/SCAI/STS Expert Consensus Document on Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2012, 59, 1200–1254.
- Ten Berg, J.; Sibbing, D.; Rocca, B.; Van Belle, E.; Chevalier, B.; Collet, J.-P.; Dudek, D.; Gilard, M.; Gorog, D.A.; Grapsa, J.; et al. Management of Antithrombotic Therapy in Patients Undergoing Transcatheter Aortic Valve Implantation: A Consensus Document of the ESC Working Group on Thrombosis and the European Association of Percutaneous Cardiovascular Interventions (EAPCI), in Collaboration with the ESC Council on Valvular Heart Disease. Eur. Heart. J. 2021, 42, 2265–2269.
- Bernelli, C.; Chieffo, A.; Montorfano, M.; Maisano, F.; Giustino, G.; Buchanan, G.L.; Chan, J.; Costopoulos, C.; Latib, A.; Figini, F.; et al. Usefulness of Baseline Activated Clotting Time—Guided Heparin Administration in Reducing Bleeding Events during Transfemoral Transcatheter Aortic Valve Implantation. JACC Cardiovasc. Interv. 2014, 7, 140–151.
- Al-Kassou, B.; Kandt, J.; Lohde, L.; Shamekhi, J.; Sedaghat, A.; Tabata, N.; Weber, M.; Sugiura, A.; Fimmers, R.; Werner, N.; et al. Safety and Efficacy of Protamine Administration for Prevention of Bleeding Complications in Patients Undergoing TAVR. JACC Cardiovasc. Interv. 2020, 13, 1471–1480.
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e72–e227.
- Ussia, G.P.; Scarabelli, M.; Mulè, M.; Barbanti, M.; Sarkar, K.; Cammalleri, V.; Immè, S.; Aruta, P.; Pistritto, A.M.; Gulino, S.; et al. Dual Antiplatelet Therapy versus Aspirin Alone in Patients Undergoing Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2011, 108, 1772–1776.
- Stabile, E.; Pucciarelli, A.; Cota, L.; Sorropago, G.; Tesorio, T.; Salemme, L.; Popusoi, G.; Ambrosini, V.; Cioppa, A.; Agrusta, M.; et al. SAT-TAVI (Single Antiplatelet Therapy for TAVI) Study: A Pilot Randomized Study Comparing Double to Single Antiplatelet Therapy for Transcatheter Aortic Valve Implantation. Int. J. Cardiol. 2014, 174, 624–627.
- Rodés-Cabau, J.; Masson, J.-B.; Welsh, R.C.; Garcia Del Blanco, B.; Pelletier, M.; Webb, J.G.; Al-Qoofi, F.; Généreux, P.; Maluenda, G.; Thoenes, M.; et al. Aspirin Versus Aspirin Plus Clopidogrel as Antithrombotic Treatment Following Transcatheter Aortic Valve Replacement with a Balloon-Expandable Valve: The ARTE (Aspirin Versus Aspirin + Clopidogrel Following Transcatheter Aortic Valve Implantation) Randomized Clinical Trial. JACC Cardiovasc. Interv. 2017, 10, 1357–1365.
- Brouwer, J.; Nijenhuis, V.J.; Delewi, R.; Hermanides, R.S.; Holvoet, W.; Dubois, C.L.F.; Frambach, P.; De Bruyne, B.; van Houwelingen, G.K.; Van Der Heyden, J.A.S.; et al. Aspirin with or without Clopidogrel after Transcatheter Aortic-Valve Implantation. N. Engl. J. Med. 2020, 383, 1447–1457.
- Ullah, W.; Zghouzi, M.; Ahmad, B.; Biswas, S.; Zaher, N.; Sattar, Y.; Pacha, H.M.; Goldsweig, A.M.; Velagapudi, P.; Fichman, D.L.; et al. Meta-Analysis Comparing the Safety and Efficacy of Single vs. Dual Antiplatelet Therapy in Post Transcatheter Aortic Valve Implantation Patients. Am. J. Cardiol. 2021, 145, 111–118.
- Guedeney, P.; Sorrentino, S.; Mesnier, J.; De Rosa, S.; Indolfi, C.; Zeitouni, M.; Kerneis, M.; Silvain, J.; Montalescot, G.; Collet, J.-P. Single Versus Dual Antiplatelet Therapy Following TAVR: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. JACC Cardiovasc. Interv. 2021, 14, 234–236.
- Asgar, A.W.; Ouzounian, M.; Adams, C.; Afilalo, J.; Fremes, S.; Lauck, S.; Leipsic, J.; Piazza, N.; Rodes-Cabau, J.; Welsh, R.; et al. 2019 Canadian Cardiovascular Society Position Statement for Transcatheter Aortic Valve Implantation. Can. J. Cardiol. 2019, 35, 1437–1448.
- Whitlock, R.P.; Sun, J.C.; Fremes, S.E.; Rubens, F.D.; Teoh, K.H. Antithrombotic and Thrombolytic Therapy for Valvular Disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th Ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141, e576S–e600S.
- Kobari, Y.; Inohara, T.; Saito, T.; Yoshijima, N.; Tanaka, M.; Tsuruta, H.; Yashima, F.; Shimizu, H.; Fukuda, K.; Naganuma, T.; et al. Aspirin Versus Clopidogrel as Single Antithrombotic Therapy After Transcatheter Aortic Valve Replacement: Insight from the OCEAN-TAVI Registry. Circ. Cardiovasc. Interv. 2021, 14, e010097.
- Angiolillo, D.J.; Fernandez-Ortiz, A.; Bernardo, E.; Alfonso, F.; Macaya, C.; Bass, T.A.; Costa, M.A. Variability in Individual Responsiveness to Clopidogrel: Clinical Implications, Management, and Future Perspectives. J. Am. Coll. Cardiol. 2007, 49, 1505–1516.
- Jimenez Diaz, V.A.; Tello-Montoliu, A.; Moreno, R.; Cruz Gonzalez, I.; Baz Alonso, J.A.; Romaguera, R.; Molina Navarro, E.; Juan Salvadores, P.; Paredes Galan, E.; De Miguel Castro, A.; et al. Assessment of Platelet REACtivity After Transcatheter Aortic Valve Replacement: The REAC-TAVI Trial. JACC Cardiovasc. Interv. 2019, 12, 22–32.
- Vavuranakis, M.A.; Kalantzis, C.; Voudris, V.; Kosmas, E.; Kalogeras, K.; Katsianos, E.; Oikonomou, E.; Siasos, G.; Aznaouridis, K.; Toutouzas, K.; et al. Comparison of Ticagrelor Versus Clopidogrel on Cerebrovascular Microembolic Events and Platelet Inhibition during Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2021, 154, 78–85.
- Sherwood, M.W.; Gupta, A.; Vemulapalli, S.; Li, Z.; Piccini, J.; Harrison, J.K.; Dai, D.; Vora, A.N.; Mack, M.J.; Holmes, D.R.; et al. Variation in Antithrombotic Therapy and Clinical Outcomes in Patients with Preexisting Atrial Fibrillation Undergoing Transcatheter Aortic Valve Replacement: Insights from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circ. Cardiovasc. Interv. 2021, 14, e009963.
- Abdul-Jawad Altisent, O.; Durand, E.; Muñoz-García, A.J.; Nombela-Franco, L.; Cheema, A.; Kefer, J.; Gutierrez, E.; Benítez, L.M.; Amat-Santos, I.J.; Serra, V.; et al. Warfarin and Antiplatelet Therapy Versus Warfarin Alone for Treating Patients with Atrial Fibrillation Undergoing Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2016, 9, 1706–1717.
- Geis, N.A.; Kiriakou, C.; Chorianopoulos, E.; Pleger, S.T.; Katus, H.A.; Bekeredjian, R. Feasibility and Safety of Vitamin K Antagonist Monotherapy in Atrial Fibrillation Patients Undergoing Transcatheter Aortic Valve Implantation. EuroIntervention 2017, 12, 2058–2066.
- Kosmidou, I.; Liu, Y.; Alu, M.C.; Liu, M.; Madhavan, M.; Chakravarty, T.; Makkar, R.; Thourani, V.H.; Biviano, A.; Kodali, S.; et al. Antithrombotic Therapy and Cardiovascular Outcomes After Transcatheter Aortic Valve Replacement in Patients with Atrial Fibrillation. JACC Cardiovasc. Interv. 2019, 12, 1580–1589.
- Nijenhuis, V.J.; Brouwer, J.; Delewi, R.; Hermanides, R.S.; Holvoet, W.; Dubois, C.L.F.; Frambach, P.; De Bruyne, B.; van Houwelingen, G.K.; Van Der Heyden, J.A.S.; et al. Anticoagulation with or without Clopidogrel after Transcatheter Aortic-Valve Implantation. N. Engl. J. Med. 2020, 382, 1696–1707.
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151.
- Patel, M.R.; Mahaffey, K.W.; Garg, J.; Pan, G.; Singer, D.E.; Hacke, W.; Breithardt, G.; Halperin, J.L.; Hankey, G.J.; Piccini, J.P.; et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 883–891.
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104.
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992.
- Pan, K.-L.; Singer, D.E.; Ovbiagele, B.; Wu, Y.-L.; Ahmed, M.A.; Lee, M. Effects of Non-Vitamin K Antagonist Oral Anticoagulants Versus Warfarin in Patients with Atrial Fibrillation and Valvular Heart Disease: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2017, 6, e005835.
- Angiolillo, D.J.; Bhatt, D.L.; Cannon, C.P.; Eikelboom, J.W.; Gibson, C.M.; Goodman, S.G.; Granger, C.B.; Holmes, D.R.; Lopes, R.D.; Mehran, R.; et al. Antithrombotic Therapy in Patients with Atrial Fibrillation Treated with Oral Anticoagulation Undergoing Percutaneous Coronary Intervention: A North American Perspective: 2021 Update. Circulation 2021, 143, 583–596.
- Overtchouk, P.; Guedeney, P.; Rouanet, S.; Verhoye, J.P.; Lefevre, T.; Van Belle, E.; Eltchaninoff, H.; Gilard, M.; Leprince, P.; Iung, B.; et al. Long-Term Mortality and Early Valve Dysfunction According to Anticoagulation Use: The FRANCE TAVI Registry. J. Am. Coll. Cardiol. 2019, 73, 13–21.
- Geis, N.A.; Kiriakou, C.; Chorianopoulos, E.; Uhlmann, L.; Katus, H.A.; Bekeredjian, R. NOAC Monotherapy in Patients with Concomitant Indications for Oral Anticoagulation Undergoing Transcatheter Aortic Valve Implantation. Clin. Res. Cardiol. 2018, 107, 799–806.
- Kalogeras, K.; Jabbour, R.J.; Ruparelia, N.; Watson, S.; Kabir, T.; Naganuma, T.; Vavuranakis, M.; Nakamura, S.; Malik, I.S.; Mikhail, G.; et al. Comparison of Warfarin versus DOACs in Patients with Concomitant Indication for Oral Anticoagulation Undergoing TAVI; Results from the ATLAS Registry. J. Thromb. Thrombolysis 2020, 50, 82–89.
- Butt, J.H.; De Backer, O.; Olesen, J.B.; Gerds, T.A.; Havers-Borgersen, E.; Gislason, G.H.; Torp-Pedersen, C.; Søndergaard, L.; Køber, L.; Fosbøl, E.L. Vitamin K Antagonists vs. Direct Oral Anticoagulants after Transcatheter Aortic Valve Implantation in Atrial Fibrillation. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 11–19.
- Kawashima, H.; Watanabe, Y.; Hioki, H.; Kozuma, K.; Kataoka, A.; Nakashima, M.; Nagura, F.; Nara, Y.; Yashima, F.; Tada, N.; et al. Direct Oral Anticoagulants Versus Vitamin K Antagonists in Patients with Atrial Fibrillation After TAVR. JACC Cardiovasc. Interv. 2020, 13, 2587–2597.
- Didier, R.; Lhermusier, T.; Auffret, V.; Eltchaninoff, H.; Le Breton, H.; Cayla, G.; Commeau, P.; Collet, J.P.; Cuisset, T.; Dumonteil, N.; et al. TAVR Patients Requiring Anticoagulation: Direct Oral Anticoagulant or Vitamin K Antagonist? JACC Cardiovasc. Interv. 2021, 14, 1704–1713.
- Tanawuttiwat, T.; Stebbins, A.; Marquis-Gravel, G.; Vemulapalli, S.; Kosinski, A.S.; Cheng, A. Use of Direct Oral Anticoagulant and Outcomes in Patients with Atrial Fibrillation after Transcatheter Aortic Valve Replacement: Insights from the STS/ACC TVT Registry. J. Am. Heart Assoc. 2021, 11, e023561.
- An, Q.; Su, S.; Tu, Y.; Gao, L.; Xian, G.; Bai, Y.; Zhan, Q.; Xu, X.; Xu, D.; Zeng, Q. Efficacy and Safety of Antithrombotic Therapy with Non-Vitamin K Antagonist Oral Anticoagulants after Transcatheter Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Ther. Adv. Chronic Dis. 2021, 12, 20406223211056730.
- Jochheim, D.; Barbanti, M.; Capretti, G.; Stefanini, G.G.; Hapfelmeier, A.; Zadrozny, M.; Baquet, M.; Fischer, J.; Theiss, H.; Todaro, D.; et al. Oral Anticoagulant Type and Outcomes After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv. 2019, 12, 1566–1576.
- Dangas, G.D.; Tijssen, J.G.P.; Wöhrle, J.; Søndergaard, L.; Gilard, M.; Möllmann, H.; Makkar, R.R.; Herrmann, H.C.; Giustino, G.; Baldus, S.; et al. A Controlled Trial of Rivaroxaban after Transcatheter Aortic-Valve Replacement. N. Engl. J. Med. 2020, 382, 120–129.
- Collet, J.P. Oral Anti-Xa Anticoagulation after Trans-Aortic Valve Implantation for Aortic Stenosis: The Randomized ATLANTIS Trial. In Proceedings of the ACC 21 Scientific Sessions, Virtual, 15 May 2021.
