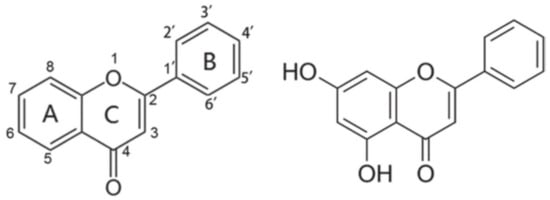
Flavonoids are polyphenolic compounds that are present in plants. They produce pharmacological actions in the peripheral and the central nervous system (CNS). They can cross the blood–brain barrier and interact with several neurotransmission systems and, thereby activating signaling pathways in specific brain structures involved in the physiopathology of anxiety and depression disorders. In particular, the flavonoid chrysin (5,7-dihydroxyflavone) has been studied for its antioxidant properties; however, its neuropharmacological effects in specific brain structures involved in the physiopathology of several neuropsychiatric disorders, such as anxiety and depression, need to be studied.
- Animal model
- antidepressant
- anxiolytic
- BDNF
- chrysin
- flavonoid
- GABAA receptor
- natural medicine
- neuropharmacology
- GABAergic system
1. Generalities of the Flavonoid Chrysin
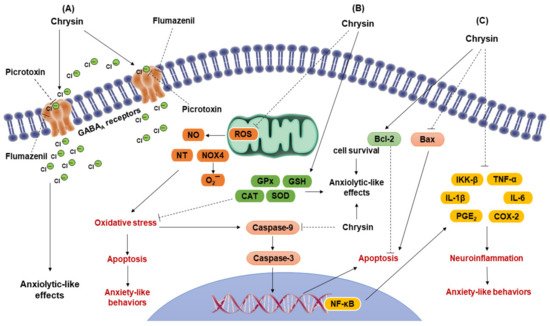
2. Anxiolytic-like Effects of Flavonoid Chrysin
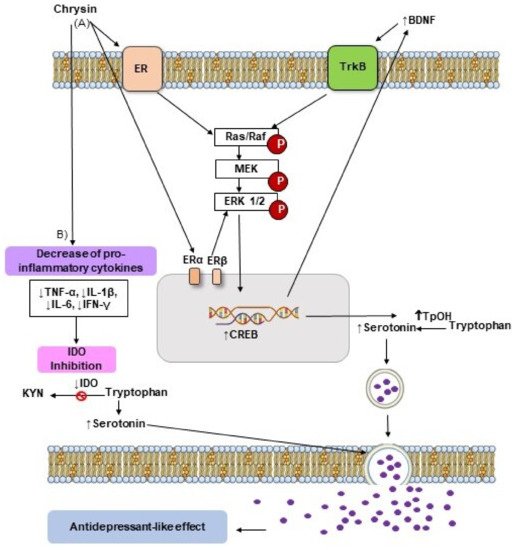
3. Antidepressant-like Effects of Flavonoid Chrysin
This entry is adapted from the peer-reviewed paper 10.3390/molecules27113551
References
- Souza, L.C.; Antunes, M.S.; Filho, C.B.; Del Fabbro, L.; de Gomes, M.G.; Goes, A.T.; Donato, F.; Prigol, M.; Boeira, S.P.; Jesse, C.R. Flavonoid chrysin prevents age-related cognitive decline via attenuation of oxidative stress and modulation of BDNF levels in aged mouse brain. Pharmacol. Biochem. Behav. 2015, 134, 22–30.
- Sathiavelu, J.; Senapathy, G.J.; Devaraj, R.; Namasivayam, N. Hepatoprotective effect of chrysin on prooxidant-antioxidant status during ethanol-induced toxicity in female albino rats. J. Pharm. Pharmacol. 2009, 61, 809–817.
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Kopustinskiene, D.M.; Simal-Gandara, J.; Bernatoniene, J.; Samarghandian, S. An updated review on the versatile role of chrysin in neurological diseases: Chemistry, pharmacology, and drug delivery approaches. Biomed. Pharm. 2021, 141, 111906.
- German-Ponciano, L.J.; Costa, B.P.D.; Feitosa, L.M.; dos Santos Campos, K.; da Silva Chaves, S.N.; Cueto-Escobedo, J.; Maximino, C. Chrysin, but not flavone backbone, decreases anxiety-like behavior in animal screens. Neurochem. Int. 2020, 140, 104850.
- Rodríguez-Landa, J.F.; Hernández-López, F.; Martínez-Mota, L.; Scuteri, D.; Bernal-Morales, B.; Rivadeneyra-Domínguez, E. GABAA/benzodiazepine receptor complex in the dorsal hippocampus mediates the effects of chrysin on anxiety-like behaviour in female rats. Front. Behav. Neurosci. 2022, 15, 789557.
- Salgueiro, J.B.; Ardenghi, P.; Dias, M.; Ferreira, M.B.; Izquierdo, I.; Medina, J.H. Anxiolytic natural and synthetic flavonoid ligands of the central benzodiazepine receptor have no effect on memory tasks in rats. Pharmacol. Biochem. Behav. 1997, 58, 887–891.
- Zanoli, P.; Avallone, R.; Baraldi, M. Behavioral characterisation of the flavonoids apigenin and chrysin. Fitoterapia 2000, 71, S117–S123.
- Filho, C.B.; Jesse, C.R.; Donato, F.; Del Fabbro, L.; de Gomes, M.G.; Goes, A.T.R.; Souza, L.C.; Giacomeli, R.; Antunes, M.; Luchese, C.; et al. Neurochemical factors associated with the antidepressant-like effect of flavonoid chrysin in chronically stressed mice. Eur. J. Pharmacol. 2016, 791, 284–296.
- Wolfman, C.; Viola, H.; Paladini, A.; Dajas, F.; Medina, J.H. Possible anxiolytic effects of chrysin, a central benzodiazepine receptor ligand isolated from Passiflora Coerulea. Pharmacol. Biochem. Behav. 1994, 47, 1–4.
- Filho, C.B.; Jesse, C.R.; Donato, F.; Del Fabbro, L.; Gomes de Gomes, M.; Rossito Goes, A.T.; Souza, L.C.; Boeira, S.P. Chrysin promotes attenuation of depressive-like behavior and hippocampal dysfunction resulting from olfactory bulbectomy in mice. Chem. Biol. Interact. 2016, 260, 154–162.
- Rodríguez-Landa, J.F.; Hernández-López, F.; Cueto-Escobedo, J.; Herrera-Huerta, E.V.; Rivadeneyra-Domínguez, E.; Bernal-Morales, B.; Romero-Avendaño, E. Chrysin (5,7-dihydroxyflavone) exerts anxiolytic-like effects through GABAA receptors in a surgical menopause model in rats. Biomed. Pharmacother. 2019, 109, 2387–2395.
- Paladini, A.C.; Marder, M.; Viola, H.; Wolfman, C.; Wasowski, C.; Medina, J.H. Flavonoids and the central nervous system: From forgotten factors to potent anxiolytic compounds. J. Pharm. Pharmacol. 1999, 51, 519–526.
- Brown, E.; Hurd, N.S.; McCall, S.; Ceremuga, T.E. Evaluation of the anxiolytic effects of chrysin, a Passiflora incarnata extract, in the laboratory rat. AANA J. 2007, 75, 333–337.
- Germán-Ponciano, L.J.; Puga-Olguín, A.; Rovirosa-Hernández, M.J.; Caba, M.; Meza, E.; Rodríguez-Landa, J.F. Differential effects of acute and chronic treatment with the flavonoid chrysin on anxiety-like behavior and Fos immunoreactivity in the lateral septal nucleus in rats. Acta Pharm. 2020, 70, 387–397.
- Ognibene, E.; Bovicelli, P.; Adriani, W.; Saso, L.; Laviola, G. Behavioral effects of 6-bromoflavanone and 5-methoxy-6,8-dibromoflavanone as anxiolytic compounds. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 128–134.
- Albert, K.; Pruessner, J.; Newhouse, P. Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology 2015, 59, 14–24.
- Rocca, W.A.; Grossardt, B.R.; Geda, Y.E.; Gostout, B.S.; Bower, J.H.; Maraganore, D.M.; de Andrade, M.; Melton, L.J. 3rd Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause 2018, 25, 1275–1285.
- Giannini, A.; Caretto, M.; Genazzani, A.R.; Simoncini, T. Neuroendocrine changes during menopausal transition. Endocrines 2021, 2, 405–416.
- Pinna, G. Allopregnanolone, the neuromodulator turned therapeutic agent: Thank you, next? Front. Endocrinol. 2020, 11, 236.
- Cueto-Escobedo, J.; Andrade-Soto, J.; Lima-Maximino, M.; Maximino, C.; Hernández-López, F.; Rodríguez-Landa, J.F. Involvement of GABAergic system in the antidepressant-like effects of chrysin (5, 7-dihydroxyflavone) in ovariectomized rats in the forced swim test: Comparison with neurosteroids. Behav. Brain Res. 2020, 386, 112590.
- Puga-Olguín, A.; Rodríguez-Landa, J.F.; Rovirosa-Hernández, M.J.; Germán-Ponciano, L.J.; Caba, M.; Meza, E.; Guillén-Ruiz, G.; Olmos-Vázquez, O.J. Long-term ovariectomy increases anxiety- and despair-like behaviors associated with lower Fos immunoreactivity in the lateral septal nucleus in rats. Behav. Brain Res. 2019, 360, 185–195.
- Lovick, T.A. GABA in the female brain—Oestrous cycle-related changes in GABAergic function in the periaqueductal grey matter. Pharmacol. Biochem. Behav. 2008, 90, 43–50.
- Rodríguez-Landa, J.F.; Guillén-Ruiz, G.; Hernández-López, F.; Cueto-Escobedo, J.; Rivadeneyra-Domínguez, E.; Bernal-Morales, B.; Herrera-Huerta, E.V. Chrysin reduces anxiety-like behavior through actions on GABAA receptors during metestrus-diestrus in the rat. Behav. Brain Res. 2021, 397, 112952.
- Rodríguez-Landa, J.F. Considerations of timing post-ovariectomy in mice and rats in studying anxiety- and depression-like behaviors associated with surgical menopause in women. Front. Behav. Neurosci. 2022, 16, 829274.
- Paul, S.M.; Pinna, G.; Guidotti, A. Allopregnanolone: From molecular pathophysiology to therapeutics. A historical perspective. Neurobiol. Stress 2020, 12, 100215.
- Medina, J.H.; Paladini, A.C.; Wolfman, C.; Levi de Stein, M.; Calvo, D.; Diaz, L.E.; Peña, C. Chrysin (5,7-di-OH-flavone), a naturally-occurring ligand for benzodiazepine receptors, with anticonvulsant properties. Biochem. Pharmacol. 1990, 40, 2227–2231.
- Goutman, J.D.; Waxemberg, M.D.; Doñate-Oliver, F.; Pomata, P.E.; Calvo, D.J. Flavonoid modulation of ionic currents mediated by GABAA and GABAc receptors. Eur. J. Pharmacol. 2003, 461, 79–87.
- Rani, N.; Bharti, S.; Bhatia, J.; Nag, T.C.; Ray, R.; Arya, D.S. Chrysin, a PPAR-γ agonist improves myocardial injury in diabetic rats through inhibiting AGE-RAGE mediated oxidative stress and inflammation. Chem. Biol. Interact. 2016, 250, 59–67.
- Park, S.S.; Park, H.S.; Kim, C.J.; Baek, S.S.; Kim, T.W. Exercise attenuates maternal separation-induced mood disorder-like behaviors by enhancing mitochondrial functions and neuroplasticity in the dorsal raphe. Behav. Brain Res. 2019, 372, 112049.
- Zhang, L.X.; Levine, S.; Dent, G.; Zhan, Y.; Xing, G.; Okimoto, D.; Kathleen Gordon, M.; Post, R.M.; Smith, M.A. Maternal deprivation increases cell death in the infant rat brain. Brain Res. Dev. Brain Res. 2002, 133, 1–11.
- Mantawy, E.M.; El-Bakly, W.M.; Esmat, A.; Badr, A.M.; El-Demerdash, E. Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. Eur. J. Pharmacol. 2014, 728, 107–118.
- Rani, N.; Bharti, S.; Bhatia, J.; Tomar, A.; Nag, T.C.; Ray, R.; Arya, D.S. Inhibition of TGF-β by a novel PPAR-γ agonist, chrysin, salvages β-receptor stimulated myocardial injury in rats through MAPKs-dependent mechanism. Nutr. Metab. 2015, 12, 11.
- Feng, X.; Qin, H.; Shi, Q.; Zhang, Y.; Zhou, F.; Wu, H.; Ding, S.; Niu, Z.; Lu, Y.; Shen, P. Chrysin attenuates inflammation by regulating M1/M2 status via activating PPARγ. Biochem. Pharmacol. 2014, 89, 503–514.
- Filho, C.B.; Jesse, C.R.; Donato, F.; Giacomeli, R.; Del Fabbro, L.; da Silva Antunes, M.; de Gomes, M.G.; Goes, A.T.R.; Boeira, S.P.; Prigol, M.; et al. Chronic unpredictable mild stress decreases BDNF and NGF levels and Na+,K+-ATPase activity in the hippocampus and prefrontal cortex of mice: Antidepressant effect of chrysin. Neuroscience 2015, 289, 367–380.
- Germán-Ponciano, L.J.; Rosas-Sánchez, G.U.; Ortiz-Guerra, S.I.; Soria-Fregozo, C.; Rodríguez-Landa, J.F. Effects of chrysin on mRNA expression of 5-HT1A and 5-HT2A receptors in the raphe nuclei and hippocampus. Rev. Bras. Farmacog. 2021, 31, 352–360.
- Bortolotto, V.C.; Pinheiro, F.C.; Araujo, S.M.; Poetini, M.R.; Bertolazi, B.S.; de Paula, M.T.; Meichtry, L.B.; de Almeida, F.P.; de Freitas Couto, S.; Jesse, C.R.; et al. Chrysin reverses the depressive-like behavior induced by hypothyroidism in female mice by regulating hippocampal serotonin and dopamine. Eur. J. Pharmacol. 2018, 822, 78–84.
- Georgieva, I.; Lepping, P.; Bozev, V.; Lickiewicz, J.; Pekara, J.; Wikman, S.; Lantta, T. Prevalence, new incidence, course, and risk factors of PTSD, depression, anxiety, and panic disorder during the COVID-19 pandemic in 11 countries. Healthcare 2021, 9, 664.
- Zhukov, D.A.; Vinogradova, E.P. Neurosteroids and depression. Neurochem. J. 2021, 15, 240–246.
- Goes, A.T.; Jesse, C.R.; Antunes, M.S.; Ladd, F.V.L.; Ladd, A.A.L.; Luchese, C.; Paroul, N.; Boeira, S.P. Protective role of chrysin on 6-hydroxydopamine-induced neurodegeneration a mouse model of Parkinson’s disease: Involvement of neuroinflammation and neurotrophins. Chem. Biol. Interact. 2018, 279, 111–120.
- Bortolotto, V.C.; Araujo, S.M.; Pinheiro, F.C.; Poetini, M.R.; Meichtry, L.B.; Fronza, M.G.; Boeira, S.P.; Savegnago, L.; Prigol, M. Chrysin restores memory deficit in hypothyroidism mice: Behavioral, neurochemical and computational approaches involving the neurotrophinergic system. J. Psychiatr. Res. 2021, 144, 225–233.
- Nakagawa, Y.; To, M.; Saruta, J.; Yamamoto, Y.; Yamamoto, T.; Shimizu, T.; Kamata, Y.; Matsuo, M.; Tsukinoki, K. Effect of social isolation stress on saliva BDNF in rat. J. Oral Sci. 2019, 61, 516–520.
