Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
In the current world situation, population and industrial growth have become major problems for energy and environmental concerns. Extremely noxious pollutants such as heavy metal ions, dyes, antibiotics, phenols, and pesticides in water are the main causes behind deprived water quality leading to inadequate access to clean water. In this connection, graphite carbon nitride (GCN or g-C3N4) a nonmetallic polymeric material has been utilized extensively as a visible-light-responsive photocatalyst for a variety of environmental applications.
- photocatalysts
- graphitic carbon nitride
- nanomaterials
- metal-doped materials
- environmental applications
1. Introduction
In attending to the needs of the rising population of the world, rapid industrialization is taking place that is leading to the environmental pollution [1][2][3][4]. One of the major sources of pollution is wastewater released mainly from chemical industries; this industrial wastewater contains high concentrations of organic waste and heavy metal ions; by their nature, these are highly poisonous and carcinogenic, causing negative impact on sustainability of water resources [5][6][7]. Recently, environmental remediation technologies such as adsorption, chemical oxidation, incineration, and biological oxidation have been used in all types of organic and toxic wastewater treatment and also have various advantages in solar energy utilization, sensing, environmental treatment, and biomedical applications. The treatment of wastewater containing the excessively disposed heavy metals and toxic organic pollutants from industries is a major challenge in the current state of the world [8][9][10]. Recently, photodegradation of organic pollutants using a variety of semiconducting materials as catalysts has progressed with high prominence for the eradication of pollutants from wastewater [11]. However, graphitic carbon nitride (GCN), a nonmetallic semiconductor having a bandgap of ~2.7 eV that can absorb blue light and high thermal and chemical stability, has provided an interest in photocatalytic applications [12]. Further, GCN is an N-richer precursor that was the first synthesized polymer through heating of chemical substances such as thiourea, urea, cyanamide, dicyandiamide, and melamine [12][13].
During the last decade, extensive study has been conducted on the creation of realistic strategies to address the energy problem and to achieve environmental restoration. High energy needs, fossil fuel depletion, and environmental degradation have all emerged as serious worldwide issues in recent years. The utilization of clean, unlimited solar energy is critical in avoiding consequences of greenhouse gases (GHGs) and ensuring future energy supplies. Every year, hundreds of scientific journals publish articles on solar energy conversion technologies, such as the photocatalytic breakdown of organic pollutants, photocatalytic water-splitting hydrogen (H2) generation, photovoltaic cells, and dye-sensitized solar cells (DSSC) [12][14]. One of the most noteworthy applications was developed by Fujishima and Honda in 1972, focused toward photoelectrochemical water splitting [15][16]. Since then, water splitting toward producing hydrogen has emerged as the most replaceable renewable energy technology with less environmental damage. GCN, similar to many other semiconducting materials, has low photocatalytic properties leading to inadequate light absorption and a significant risk of photogenerated electron and hole pair recombination. Generally, the cocatalyst has been widely examined to generate the composites with GCN for oxidation and reduction of active sites, trapping charged carriers, and inhibiting photogenerated electron-hole pair recombination [17].
Elemental doping can significantly alter GCN’s bandgap structure. This increases the hole and electron separation caused by the light. Wang and coworkers observed that the F-doping to the GCN matrix results in C-F bond formation, which can boost the light absorption range [18]. In another study, metals such as Fe were loaded onto GCN (Fe-GCN) and used the π-π structure to use the Fenton-like reaction. This suggests that GCN has excellent catalytic efficiency in the realm of cleaning. Here, Fe-GCN was synthesized to investigate the efficiency of challenging wastewater treatment using a heterogeneous photocatalysis–Fenton system. It also helps to separate photoelectrons from holes. In complex wastewater, the product delivered efficient organic matter removal [19][20]. In these cases, the s-triazine (C3N3) rings of GCN were certified as being the most stable in the environment with typical tri-s-triazine (C6N7) units.
Various studies of GCN-based photocatalysts dealing the modification and synthesis, and their uses in environmental and energy challenges, are currently available and only a few publications have emphasized mostly on wide-ranging characteristics and g-GCN-based photocatalyst performance enhancement [21][22]. In the end, it becomes appropriate to provide a rather complete and fully updated evaluation of the most recent achievements in metal-doped GCN-based photocatalysts for heterogeneous photocatalysis. Potential applications of GCN photocatalysts are briefly discussed in here. These following content also introduce the new GCN-based architectures and materials for further developments with better use of photocatalytic efficiency and solar energy, and it helps to show the challenges for the worldwide GCN-based photocatalysts utilization in the storage and production of sustainable energy from renewable resources. These following content also introduce a few new ideas concerning architectures in developing new materials for the fuel cells, emitting devices, solar cells, sensing devices, and batteries for different sustainable-energy-associated fields. Figure 1 depicts design criteria for GCN-based photocatalysts focusing on various photocatalytic phases.
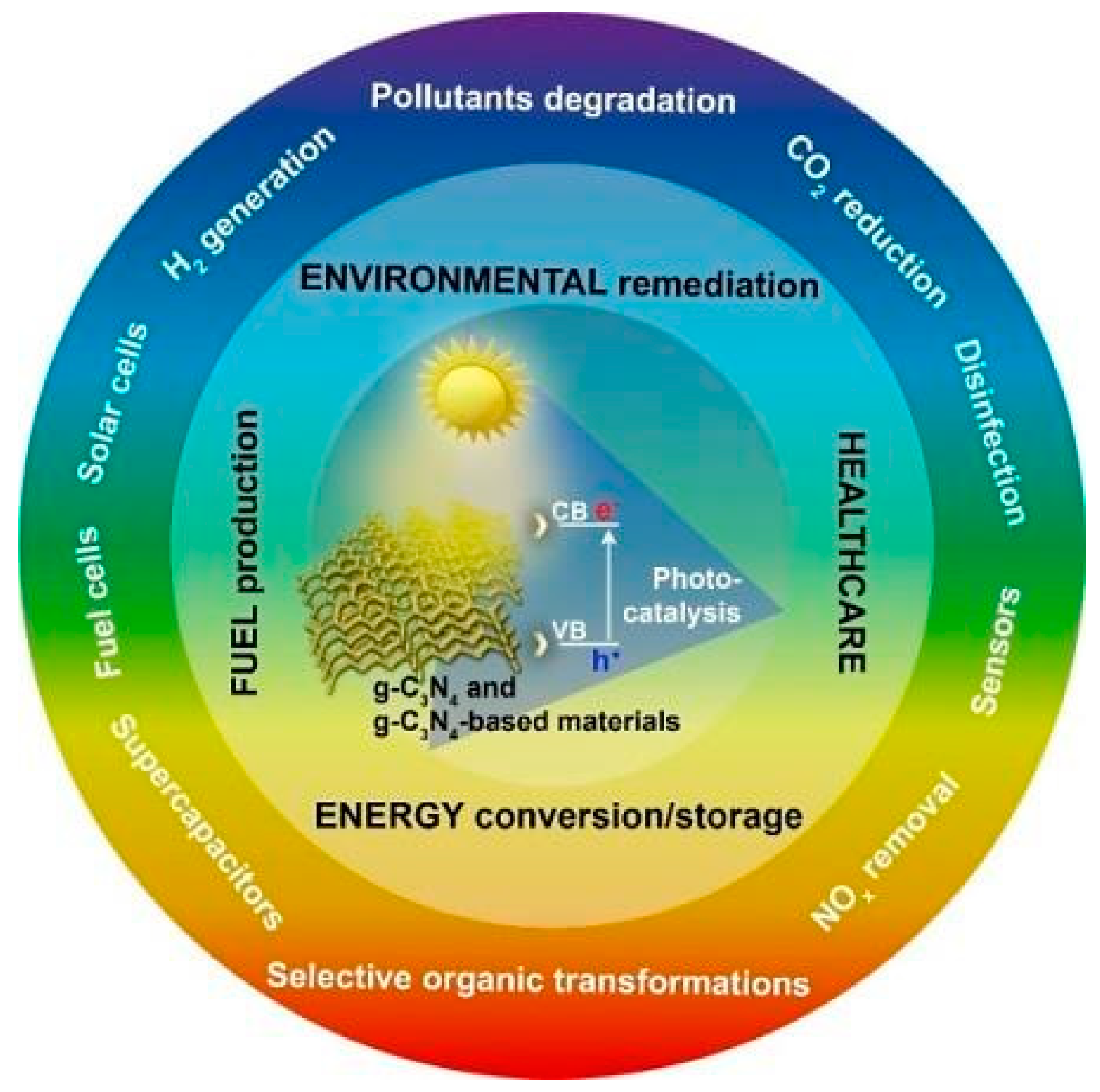
Figure 1. Considerations for GCN-based photocatalysts in various applications.
General Facts/Features of GCN
In the 1830s, GCN was first found by Berzelius and recognized by Liebig as one of the earliest synthetic polymers [24]. Because of its unknown chemical structure, insolubility, and chemical inertness, it was not widely employed until now. Wang et al.’s theoretical predictions highlighted the properties of GCN and GCN uses for photocatalysis [25], which is a pioneering work on photocatalytic H2 evolution. The framework structure, similar to graphene, is composed of N-bridged poly aromatic heptazine π-conjugated defect-rich units that create sheets with very strong covalent C-N connections. Additionally, its properties include the ease of changing the bandgap, cost-effective synthesis, nontoxicity, absorption of near-visible light, thermal and chemical stability, and high excitation recombination rates. GCN’s practical application/actual use, however, is limited due to detriments such as low specific surface area, surface inertness, weak charge transport and kinetics, and high excitation recombination levels. To overcome these flaws, doping to strengthen the heterostructure production are commonly used to improve the electronic structure, exfoliation to increase surface area, porous architecture construction to promote surface characteristics, enhance charge transport kinetics, etc.
2. The Catalytic Mechanism of Photocatalysts Based on GCN Heterogeneous
Most GCN research has focused on narrowing the energy bandgap while simultaneously decreasing charge photogenerated electron-hole pair’s recombination: (i) doping metallic elements into GCN as electron entrapment centers, generating highly oxidized holes, (ii) creating heterojunctions, such as metal–semiconductor, semiconductor–semiconductor to efficiently divide holes and electrons [26]. Recent research has shown that incorporating the GCN metal ions into the polymerization network enhances lifespan and charge carrier mobility while also lowering the material bandgap. Tri-s-triazine moieties coupled by three-fold nitrogen bridges provide a wide area in the GCN network having six lone pairs of electrons on nitrogen atoms, and these can help in optimal coordination for transition metal ion accommodation. Figure 2 depicts the schematic diagram of GCN photocatalytic performance.
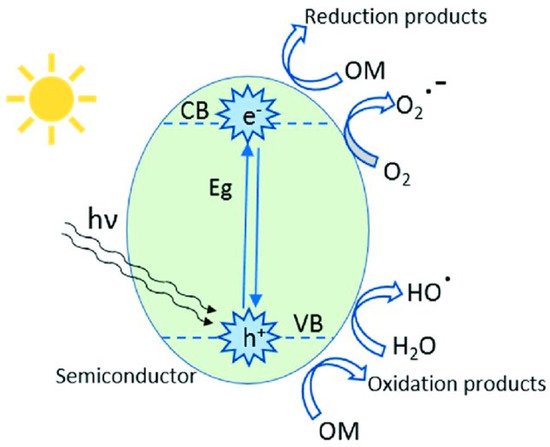
Figure 2. Schematic representation of heterogeneous photocatalysis mechanism.
Most GCN research has focused on narrowing the energy bandgap while simultaneously decreasing charge photogenerated electron-hole pair’s recombination: (i) doping metallic elements into GCN as electron entrapment centers, generating highly oxidized holes (ii) creating heterojunctions, like metal-semiconductor, semiconductor-semiconductor to efficiently divide holes and electrons [26]. Recent research has shown that incorporating the GCN metal ions into the polymerization network enhances lifespan and charge carrier mobility while also lowering the material bandgap. Tri-s-triazine moieties coupled by three-fold nitrogen bridges provide a wide area in the GCN network having six lone pairs of electrons on nitrogen atoms and these can help in optimal coordination for transition metal ion accommodation. Figure 3 and Figure 4 show the fabrication of Fe-doped GCN (Figure 3) and its critical role in photocatalytic methanol oxidation (Figure 4).
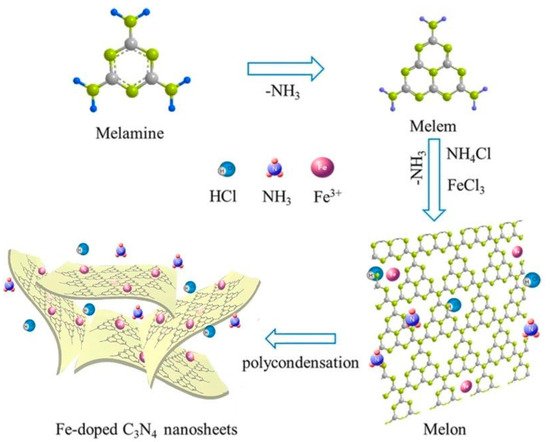
Figure 3. Fabrication of Fe-doped GCN.
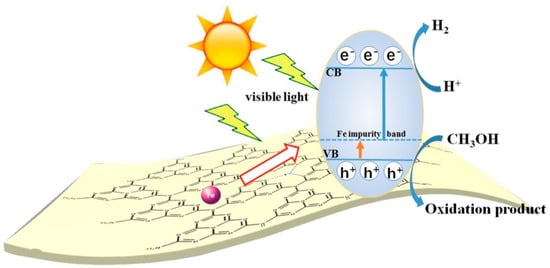
Figure 4. The charge mobility in Fe-doped GCN photocatalytic performance.
Since Wang et al. (2009) discovered a change in the GCN functionality when doped with certain metals, including Fe [29], Fe-doped GCN nanosheets have been explored as a means to develop extremely efficient photocatalysts and to investigate the mechanism that increases their photocatalytic activity. Tonda et al. (2014) [30] investigated Fe-doped GCN nanosheets produced from melamine and FeCl3 using a two-step process. The involvement of Fe3+ as a photogenerated electron trap explains the large increase in photocatalytic performance for RbH degradation. Recently, Ma et al. [31] published an article in 2019 on the Fe-doped GCN photocatalyst produced by single-step melamine thermal condensation and iron nitrate nanohydrate, emphasizing the importance of the Fe3+/Fe2+ pair in the photocatalytic activity. Interestingly, research on Fe− and P-codoped GCN revealed a considerable increase in photocatalytic efficiency. Although increased photocatalytic efficiency and mechanism in Fe-doped GCN material are still being verified, it has made a significant contribution to the detection of photocatalysts in general and solar energy conversion materials in particular [32].
2.1. Benefits and Drawbacks of g-GCN-Based Photocatalysts
Based on the study of bandgap and nanostructures, both are very important for photocatalytic applications. Figure 4 depicts the GCN bandgap structures along with various standard potentials of common redox processes at pH 7. From Figure 4, it can be seen that GCN has a modest bandgap of 2.7 eV, which corresponds to 460 nm optical wavelength, making it visible-light active. The potential and thermodynamic losses are considered in the photocatalytic process, and the 2.7 eV bandgaps fall, allowing for both visible-light absorption and water splitting. From the Figure 4, it is noted that the top CB level of GCN is more negative than those potentials and conventional inorganic semiconductor of CO2-reduction, oxygen reduction reactions, and hydrogen evolution, which suggests GCN possesses photogenerated electrons with a high thermodynamic driving force that can reduce different types of small substances, such as O2, H2O, and CO2 [33]. As a result, the GCN band structure makes it suited to a wide variety of applications, including reduction of carbon, photocatalytic water splitting, disinfection, and organic synthesis.
GCN-based photocatalytic efficiency is predominantly determined by crystal structure, electrochemical and photoelectrochemical stability, optical and surface physicochemical adsorption, and electronic characteristics of GCN. As a result, a basic understanding and control of these structural and chemical factors allows for scalable GCN-based composite photocatalyst production. This includes better photocatalytic behavior, which is useful for developing some robust GCN-based material systems for functional photocatalytic implementation and fundamental understanding of photocatalytic enhancement mechanisms at the single-atom level [34].
2.2. Photocatalytic Hydrogen Gas (H2)
H2 gas production has recently attracted widespread research interest. Although fossil fuel energy has greater heat energy production, fossil fuels are being depleted. Because of its clean reactions and simplicity, conventional solar energy remains the most promising technology for the mechanisms of water splitting to generate hydrogen. She and colleagues [35] described the 2D-Fe2O3/GCN Z-scheme catalyst synthesis. From the literature, their report shows hydrogen evolution activity was greatly increased in hybrids with Fe2O3 nanostructures, which can reach 31,400 mol g−1 h−1 for Fe2O3/2D GCN (Fe2O3-loading 3.8 wt%). According to these studies, heterostructure carbon nitride semiconductors for hydrogen production exhibit high photocatalytic efficiency [36]. Table 1 summarizes the various g-GCN heterojunction photocatalytic hydrogen production investigations. The difficulty of splitting oxygen-containing products and hydrogen (hydrogen storage mechanism) in photocatalytic hydrogen (H2) synthesis is impeded by the proximity to reduction–oxidation sites. As a result, it is more difficult to obtain photogenerated electrons and holes at the oxidation and reduction sites in the proposed photocatalyst, which could result in oxygen- and hydrogen-containing products reversing reactions or even causing explosive damage. To overcome this obstacle, researchers have searched for separately produced H2 from oxygen products while keeping the tight spacing between oxidation and reduction sites, which is critical for photoinduced charge transfer. Graphene sandwich devices with photocatalytic hydrogen production and storage capabilities were created by Li Yang and colleagues in 2017 [37].
Table 1. Hydrogen production study by differing GCN heterostructure.
| Photocatalytic Material | Light Source | Performance |
|---|---|---|
| GCN/Au/CdS | 300 W Xenon lamp | 530 μmol after 5 h |
| C,N-TiO2/GCN | 300 W Xenon arc lamp | 39.18 mmol h−1 g−1 |
| GCN/WS2 | 300 W Xenon arc lamp | 101 μmol h−1 g−1 |
| WO3/GCN | Artificial solar light | 110 μmol h−1 g−1 |
| WO3/GCN | 300 W Xenon | 1853 μmol h−1 g−1 |
| Bi2MoO6/GCN | 300 W Xenon lamp | 563.4 μmol h−1 g−1 |
This is performed not just to avoid the backward reaction, but also to make it possible for the storage of created H2 on the surface, with a 5.2 wt% storage rate, which is very near to that of the U.S. Department of Energy’s guidelines (6.5 percent by weight). Xijun Wang’s group also used first principle calculations to manufacture a carbon–quantum-dot/GCN hybrid with a strong capability to isolate H2 from the oxygen in photocatalytic water splitting. The protons were only allowed to penetrate the graphene inner layer to create H2 in their work [39]. The H2 gas created was then encapsulated in the photocatalyst’s inner layer; this also prevented the process from reversing and increased the H2 availability that was generated.
2.3. CO2 Reduction
The ecosystem, notably the atmosphere, has suffered as a result of population increase and industrialization. CO2 emissions have recently remained the most pressing issue in the cosmos. CO2 produced by fuel combustion at all levels, from home to industry, has significantly facilitated the rise of atmospheric air pollution, which is largely responsible for the current global warming crisis [40]. To reduce CO2 emissions, various strategies have been devised. SDG 7 calls for the use of clean, renewable energy by lowering CO2 emissions in the atmosphere. However, rising fuel use and industrial production continue to contribute significantly to CO2 emissions (Table 2). Technologies to degrade the CO2 produced have been developed. Photocatalytic reactions, for example, have been shown to be one of the most promising technologies for CO2 removal. Sheng Zhou and colleagues [41] described a simple in situ fabricated GCN-N-TiO2 heterojunction as an efficient photocatalyst for CO2 reduction. Thermal treatment of well-mixed Ti (OH)4 and urea with varying mass ratios in an alumina crucible resulted in composites of GCN and N-doped TiO2 (such as GCN-N-TiO2). To obtain the product, this mixture was heated at 550 °C for 3 h, followed by heating at 580 °C for 3 h at 5 °C per min rate. To remove traces of adsorbed alkaline and sulfate species, the product was washed several times with 0.1 M HNO3 followed by distilled water. Finally, the material obtained was dried overnight at 80 °C. The photocatalytic CO2 reduction property of the fabricated material was investigated in a closed gas-circulation system using water vapor and CO2 under simulated light irradiation. Because it facilitates the parting of electrons that are induced by light from holes, the heterojunction between GCN and nitrogen-doped TiO2 displayed high activity.
Table 2. Studies of GCN heterojunctions on carbon dioxide (CO2) reduction.
These findings show that when compared to its precursors, the hereto structured GCN semiconductor has a high photocatalytic performance in CO2 reduction. More investigations on heterojunction for CO2 photocatalytic reduction are presented in Table 2.
This entry is adapted from the peer-reviewed paper 10.3390/nano12101754
References
- Sharma, G.D.; Shah, M.I.; Shahzad, U.; Jain, M.; Chopra, R. Exploring the nexus between agriculture and greenhouse gas emissions in BIMSTEC region: The role of renewable energy and human capital as moderators. J. Environ. Manag. 2021, 297, 113316.
- Venkateswarlu, K. Ashes from organic waste as reagents in synthetic chemistry: A review. Environ. Chem. Lett. 2021, 19, 3887–3950.
- Singh, R.S.; Singh, T.; Hassan, M.; Larroche, C. Biofuels from inulin-rich feedstocks: A comprehensive review. Bioresour. Technol. 2022, 346, 126606.
- Appa, R.M.; Naidu, B.R.; Venkateswarlu, D.; Hanafiah, M.M.; Lakkaboyana, S.K.; Lakshmidevi, J.; Venkateswarlu, K. Water extract of pomegranate ash–I2 as sustainable system for external oxidant/metal/catalyst-free oxidative iodination of (hetero)arenes. Green Chem. Lett. Rev. 2021, 14, 700–712.
- Vijitha, R.; Nagaraja, K.; Hanafiah, M.M.; Rao, K.M.; Venkateswarlu, K.; Lakkaboyana, S.K.; Rao, K.S.V. Fabrication of eco-friendly polyelectrolyte membranes based on sulfonate grafted sodium alginate for drug delivery, toxic metal ion removal and fuel cell applications. Polymers 2021, 13, 3293.
- Lee, K.E.; Hanafiah, M.M.; Halim, A.A.; Mahmud, M.H. Primary treatment of dye wastewater using aloe vera-aided aluminium and magnesium hybrid coagulants. Procedia Environ. Sci. 2015, 30, 56–61.
- Vijitha, R.; Reddy, N.S.; Nagaraja, K.; Vani, T.J.S.; Hanafiah, M.M.; Venkateswarlu, K.; Lakkaboyana, S.K.; Rao, K.S.V.K.; Rao, K.M. Fabrication of polyelectrolyte membranes of pectin graft-copolymers with PVA and their composites with phosphomolybdic acid for drug delivery, toxic metal ion removal, and fuel cell applications. Membranes 2021, 11, 792.
- Vallinayagam, S.; Rajendran, K.; Lakkaboyana, S.K.; Soontarapa, K.; Remya, R.R.; Sharma, V.K.; Kumar, V.; Venkateswarlu, K.; Koduru, J.R. Recent developments in magnetic nanoparticles and nano-composites for wastewater treatment. J. Environ. Chem. Eng. 2021, 9, 106553.
- Ismael, M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis. J. Alloys Compd. 2020, 846, 156446.
- Jiang, L.; Yang, J.; Zhou, S.; Yu, H.; Liang, J.; Chu, W.; Li, H.; Wang, H.; Wu, Z.; Yuan, X. Strategies to extend near-infrared light harvest of polymer carbon nitride photo catalysts. Coord. Chem. Rev. 2021, 439, 213947.
- Zada, A.; Qu, Y.; Ali, S.; Sun, N.; Lu, H.; Yan, R.; Zhang, X.; Jing, L. Improved visible-light activities for degrading pollutants on TiO2/g-C3N4 nanocomposites by decorating SPR Au nanoparticles and 2,4-dichlorophenol decomposition path. J. Hazard. Mater. 2018, 342, 715–723.
- Yang, J.; Wang, H.; Jiang, L.; Yu, H.; Zhao, Y.; Chen, H.; Yuan, X.; Liang, J.; Li, H.; Wu, Z. Defective polymeric carbon nitride: Fabrications, photocatalytic applications and perspectives. Chem. Eng. J. 2022, 427, 130991.
- Yu, Y.; Zhou, Q.; Wang, J. The ultra-rapid synthesis of 2D graphitic carbon nitride nanosheets via direct microwave heating for field emission. Chem. Commun. 2016, 52, 3396–3399.
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176.
- Kavitha, R.; Nithya, P.M.; Kumar, S.G. Noble metal deposited graphitic carbon nitride-based heterojunction photocatalysts. Appl. Surf. Sci. 2020, 508, 145142.
- Qi, K.; Liu, S.; Zada, A. Graphitic carbon nitride, a polymer photocatalyst. J. Taiwan Inst. Chem. Eng. 2020, 109, 111–123.
- Palani, G.; Arputhalatha, A.; Kannan, K.; Lakkaboyana, S.K.; Hanafiah, M.M.; Kumar, V.; Marella, R.K. Current trends in the application of nanomaterials for the removal of pollutants from industrial wastewater treatment—A review. Molecules 2021, 26, 2799.
- Cao, S.; Yu, J. g-C3N4-based photocatalysts for hydrogen generation. J. Phys. Chem. Lett. 2014, 5, 2101–2107.
- Jiang, L.; Zhou, S.; Yang, J.; Wang, H.; Yu, H.; Chen, H.; Zhao, Y.; Yuan, X.; Chu, W.; Li, H. Near-infrared light responsive TiO2 for efficient solar energy utilization. Adv. Funct. Mater. 2022, 32, 2108977.
- Ge, L.; Han, C.; Xiao, X.; Guo, L.; Li, Y. Enhanced visible-light photocatalytic hydrogen evolution of sulfur-doped polymeric g-C3N4 photocatalysts. Mater. Res. Bull. 2013, 48, 3919–3925.
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: A review. Appl. Catal. B Environ. 2017, 217, 388–406.
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric graphitic carbon nitride for heterogeneous photocatalysis. ACS Catal. 2012, 2, 1596–1606.
- Vasiljević, J.; Jerman, I.; Simončič, B. Graphitic Carbon Nitride as a New Sustainable Photocatalyst for Textile Functionalization. Polymers 2021, 13, 2568.
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418.
- Ye, S.; Wang, R.; Wu, M.-Z.; Yuan, Y.-P. A review on g-C3N4 for photocatalytic water splitting and CO2 reduction. Appl. Surf. Sci. 2015, 358, 15–27.
- Patsoura, A.; Kondarides, D.I.; Verykios, X.E. Photocatalytic degradation of organic pollutants with simultaneous production of hydrogen. Catal. Today 2007, 124, 94–102.
- Danfá, S.; Martins, R.C.; Quina, M.J.; Gomes, J. Supported TiO2 in Ceramic Materials for the Photocatalytic Degradation of Contaminants of Emerging Concern in Liquid Effluents: A Review. Molecules 2021, 26, 5363.
- Gao, J.; Wang, Y.; Zhou, S.; Lin, W.; Kong, Y. A facile one-step synthesis of Fe-doped g-C3N4 nanosheets and their improved visible-light photocatalytic performance. ChemCatChem 2017, 9, 1708–1715.
- Xu, Q.; Jiang, C.; Cheng, B.; Yu, J. Enhanced visible-light photocatalytic H2-generation activity of carbon/g-C3N4 nanocomposites prepared by two-step thermal treatment. Dalton Trans. 2017, 46, 10611–10619.
- Zhu, Z.; Lu, Z.; Wang, D.; Tang, X.; Yan, Y.; Shi, W.; Wang, Y.; Gao, N.; Yao, X.; Dong, H. Construction of high-dispersed Ag/Fe3O4/g-C3N4 photocatalyst by selective photo-deposition and improved photocatalytic activity. Appl. Catal. B Environ. 2016, 182, 115–122.
- Theerthagiri, J.; Senthil, R.A.; Priya, A.; Madhavan, J.; Michael, R.J.V.; Ashokkumar, M. Photocatalytic and photoelectrochemical studies of visible-light active α-Fe2O3–g-C3N4 nanocomposites. RSC Adv. 2014, 4, 38222–38229.
- Cao, J.; Zhao, Y.; Lin, H.; Xu, B.; Chen, S. Ag/AgBr/g-C3N4: A highly efficient and stable composite photocatalyst for degradation of organic contaminants under visible light. Mater. Res. 2013, 48, 3873–3880.
- Li, J.; Yin, Y.; Liu, E.; Ma, Y.; Wan, J.; Fan, J.; Hu, X. In situ growing Bi2MoO6 on g-C3N4 nanosheets with enhanced photocatalytic hydrogen evolution and disinfection of bacteria under visible light irradiation. J. Hazard. Mater. 2017, 321, 183–192.
- Hitam, C.N.C.; Jalil, A.A. A review on an exploration of Fe2O3 photocatalyst towards the degradation of dyes and organic contaminants. J. Environ. Manag. 2020, 258, 110050.
- Han, X.; Xu, D.; An, L.; Hou, C.; Li, Y.; Zhang, Q.; Wang, H. WO3/g-C3N4 two-dimensional composites for visible-light-driven photocatalytic hydrogen production. Int. J. Hydrog. Energy 2018, 43, 4845–4855.
- Li, W.; Feng, C.; Dai, S.; Yue, J.; Hua, F.; Hou, H. Fabrication of sulfur-doped g-C3N4/Au/CdS Z-scheme photocatalyst to improve the photocatalytic performance under visible light. Appl. Catal. B Environ. 2015, 168–169, 465–471.
- Yu, W.; Xu, D.; Peng, T. Enhanced photocatalytic activity of g-C3N4 for selective CO2 reduction to CH3OH via facile coupling of ZnO: A direct Z-scheme mechanism. J. Mater. Chem. A 2015, 3, 19936–19947.
- Huang, R.; Wu, J.; Zhang, M.; Liu, B.; Zheng, Z.; Luo, D. Strategies to enhance photocatalytic activity of graphite carbon nitride-based photocatalysts. Mater. Des. 2021, 210, 110040.
- Li, X.; Yu, J.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636.
- Qu, X.; Hu, S.; Bai, J.; Li, P.; Lu, G.; Kang, X. A facile approach to synthesize oxygen doped g-C3N4 with enhanced visible-light activity under anoxic conditions via oxygen-plasma treatment. New J. Chem. 2018, 42, 4998–5004.
- Tonda, S.; Kumar, S.; Kandula, S.; Shanker, V. Fe-doped and-mediated graphitic carbon nitride nanosheets for enhanced photocatalytic performance under natural sunlight. J. Mater. Chem. A 2014, 2, 6772–6780.
- Wang, J.-C.; Yao, H.-C.; Fan, Z.-Y.; Zhang, L.; Wang, J.-S.; Zang, S.-Q.; Li, Z.-J. Indirect Z-scheme BiOI/g-C3N4 photocatalysts with enhanced photoreduction CO2 activity under visible light irradiation. ACS Appl. Mater Interfaces 2016, 8, 3765–3775.
- He, Y.; Zhang, L.; Fan, M.; Wang, X.; Walbridge, M.L.; Nong, Q.; Wu, Y.; Zhao, L. Z-scheme SnO2-x/g-C3N4 composite as an efficient photocatalyst for dye degradation and photocatalytic CO2 reduction. Sol. Energy Mater. Sol. Cells 2015, 137, 175–184.
- Yan, J.; Wu, H.; Chen, H.; Pang, L.; Zhang, Y.; Jiang, R.; Li, L.; Liu, S. One-pot hydrothermal fabrication of layered β-Ni(OH)2/g-C3N4 nanohybrids for enhanced photocatalytic water splitting. Appl. Catal. B Environ. 2016, 194, 74–83.
This entry is offline, you can click here to edit this entry!
