Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Thymoquinone is isolated from Nigella sativa with a molecular weight of 164.2 g/mol. A series of cutting-edge research works have demonstrated the health-promoting and disease-ameliorating roles of thymoquinone.
- anticancer
- thymoquinone
- anti-metastasis
- signaling networks
1. Introduction
Deregulated cell signaling pathways play fundamental roles in re-shaping multiple facets of cancer, encompassing a wide range of functions that include the early initiation stage through to the metastatic stage, drug resistance, loss of apoptosis, and genetic/epigenetic inactivation [1][2][3][4]. Ground-breaking discoveries associated with the extra-ordinary pharmaceutical and medicinal values of natural products have catalyzed outstanding breakthroughs in drug development, consequently resulting in the rapid maturation of generalizable chemical platforms for the multi-targeting of previously undruggable oncogenic proteins [5][6][7][8][9].
Thymoquinone is isolated from Nigella sativa with a molecular weight of 164.2 g/mol. A series of cutting-edge research works have demonstrated the health-promoting and disease-ameliorating roles of thymoquinone [10][11][12][13][14]. Although different research teams have reviewed cancer chemopreventive role of thymoquinone [15][16][17][18][19], herein summarize mechanistic insights gained through cell culture studies and xenografted mice. Also, researchers provide a comprehensive overview of the fast-evolving field of pharmacological targeting of signaling pathways by thymoquinone in cancer and discuss the possibilities associated with targeting JAK/STAT, Wnt/β-catenin, PI3K/AKT/mTOR and NF-κB for drug development in a wide range of oncology settings. The researchers underline the knowledge gaps, identify key challenges, and make recommendations on how to rationally accelerate laboratory findings into clinically effective therapeutics.
2. Regulation of JAK/STAT
A multimodule gene regulatory network mediates the complex process, involving signaling events and regulation of target genes, which modulates carcinogenesis and metastasis. The JAK/STAT pathway has been reported to be extensively involved in carcinogenesis and the spread of cancer cells to distant organs [20][21][22]. This section mainly deals with the thymoquinone-mediated inhibitory effects on the JAK/STAT pathway.
Thymoquinone inhibited the JAK2-mediated phosphorylation of STAT3 on the 727th serine residue in SK-MEL-28 cells. Importantly, levels of cyclin D1, D2, and D3 were reported to be reduced in STAT3-depleted SK-MEL-28 cells. Intraperitoneally administered thymoquinone caused tumor shrinkage in mice inoculated with SK-MEL-28 cells [23].
Gamma knife has been shown to be an effective treatment against brain metastasis from melanoma. Brain metastasis commonly occurs in patients suffering from metastatic malignant melanoma. Essentially, conversion of transient remissions to stable cures remains an overarching goal for clinical investigators of melanoma. Gamma knife and thymoquinone combinatorially enhanced the survival rates of C57BL/6J mice with intra-cerebral B16-F10 melanoma [24].
Furthermore, thymoquinone has also been reported to increase the survival rates in C57BL/6J mice with intracerebral B16-F10 melanoma cells. The JAK2/STAT3 pathway is inactivated by thymoquinone in B16-F10 melanoma cells. Collectively, these results clearly indicate that pharmacological targeting of melanoma brain metastasis by thymoquinone needs to be tested more comprehensively [25].
Renal cell carcinoma cells have high levels of anti-apoptotic proteins (Chae). It has also been noted that thymoquinone blocked the JAK2/STAT3-mediated upregulation of BCL-2, survivin, and cyclin D2 in Caki-1 cells (Figure 1). Intraperitoneal injections of thymoquinone hampered the growth of Caki-1 cell xenografts in nude mice [26].
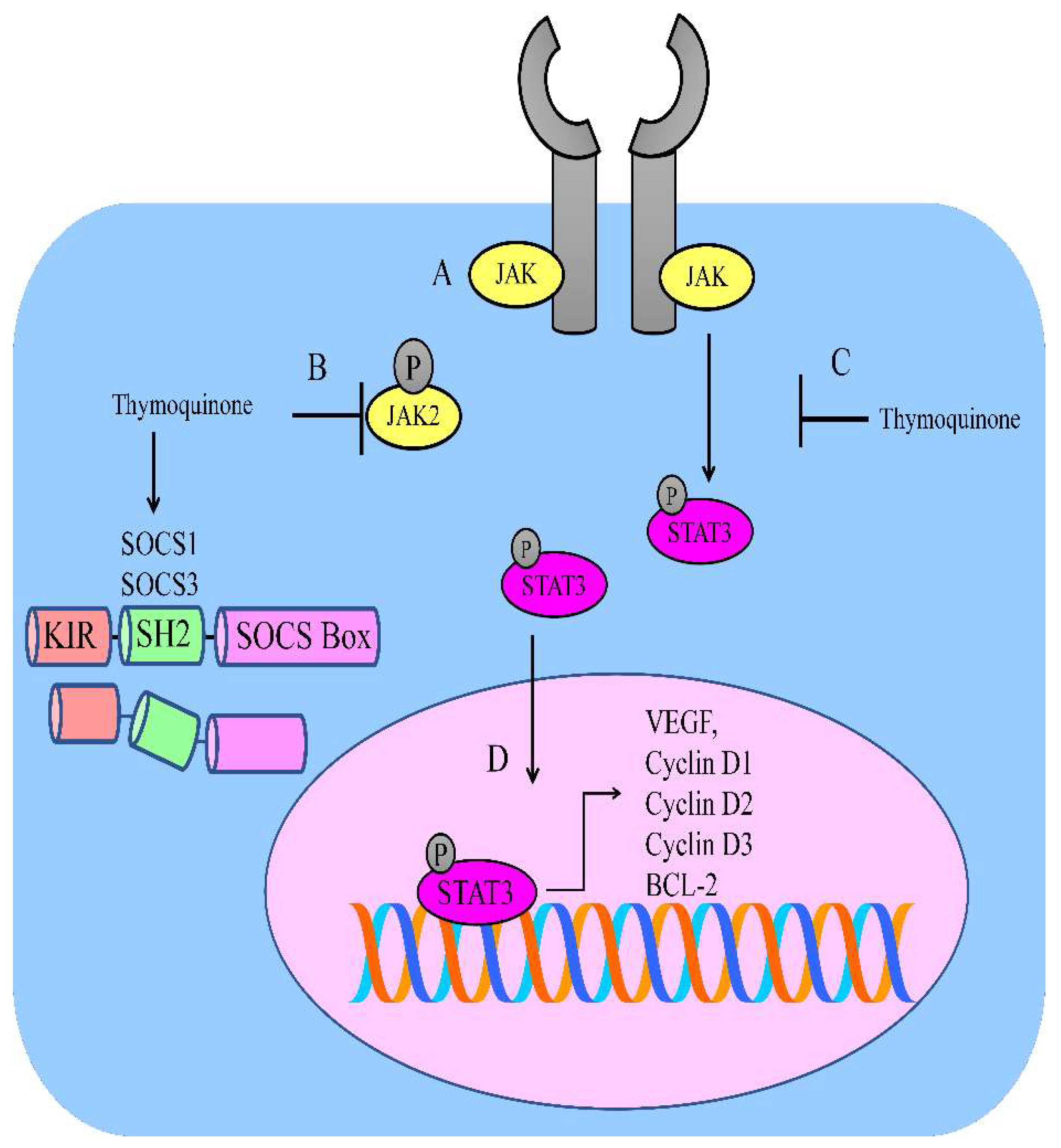 Figure 1. (A) JAK/STAT-mediated signaling is regulated by thymoquinone. (B,C) Thymoquinone inhibited JAK2 and STAT3. Thymoquinone stimulated the expression of SOCS1 and SOCS3. (D) STAT3-mediated upregulation of VEGF; BCL-2; and cyclin D1, D2, and D3.
Figure 1. (A) JAK/STAT-mediated signaling is regulated by thymoquinone. (B,C) Thymoquinone inhibited JAK2 and STAT3. Thymoquinone stimulated the expression of SOCS1 and SOCS3. (D) STAT3-mediated upregulation of VEGF; BCL-2; and cyclin D1, D2, and D3.Similarly, intraperitoneally administered thymoquinone impaired tumor growth rates in NSG mice xenotransplanted with epidermoid carcinoma A431 cells. Importantly, p53 is negatively regulated by MDM2. It was noted that thymoquinone increased p53 levels by simultaneous suppression in the level of MDM2 [27].
Thymoquinone inactivated the JAK2/STAT3 pathway in gastric HGC27 cancer cells. Thymoquinone also suppressed STAT3 target genes, such as survivin, VEGF, cyclin D, and BCL-2 (Figure 1). Intraperitoneally administered injections of thymoquinone induced tumor shrinkage in xenografted models [28].
Thymoquinone and cisplatin induced the regression of tumor xenografts. Importantly, p-STAT3 levels were noted to be profoundly reduced in the tumor tissues of thymoquinone and cisplatin-treated mice [29].
Evidence suggests that thymoquinone is efficient against acute myeloid leukemia. Thymoquinone chemically interacted with the active pockets of JAK2, STAT3, and STAT5 and inhibited their activities. Over the past two decades, following the discovery of the SOCS protein family, a wealth of knowledge has uncovered the functions and structures of SOCS proteins. SOCS proteins are negative-feedback regulators and a thymoquinone-mediated increase in SOCS proteins is necessary for inactivation of STAT proteins. Thymoquinone stimulated the expression of SHP-1, SOCS1, and SOCS3 in MV4–11 cells (Figure 1) [30].
3. Wnt/β-Catenin Signaling
With the advancements in sequencing technologies and detailed structural characterization of the cancer genome, it is apparent that WNT pathway mutations frequently occur in various cancers. β-Catenin is widely acclaimed as the principal transducer of canonical WNT signals to the nucleus. The orchestrated co-operation between cell surface mechanics and intracellular signaling has significant impacts on biological processes. In the absence of WNT ligands, β-catenin is phosphorylated by GSK3β, ubiquitylated by β-TrCP, and marked for degradation. The classical pathway is switched “on” upon binding of WNT ligands to frizzled receptors and LRP co-receptors, which results in stabilization and accumulation of β-catenin [31][32][33][34].
Thymoquinone dose-dependently inhibited nuclear accumulation of β-catenin [35]. Levels of β-catenin and Wnt/β-catenin target genes, such as c-Myc, matrix metalloproteinase-7, and Met, were found to be reduced in thymoquinone-treated bladder cancer cells. Importantly, β-catenin overexpression drastically abrogated the repressive effects of thymoquinone on the epithelial-to-mesenchymal transition by enhancing the levels of N-cadherin and vimentin. Intraperitoneally injected thymoquinone caused regression of tumor mass in xenografted mice. Moreover, pulmonary metastases models were generated by injections of T24-L-tagged luciferase for evaluation of the repressive effects of thymoquinone on distant metastasis. Moreover, bioluminescence imaging clearly indicated that pulmonary metastases were inhibited considerably by thymoquinone. Thymoquinone has been shown to significantly inhibit the foci of lung metastasis [35].
The MITF promoter present in close vicinity to the common downstream exon is known as the M promoter and expressed selectively in melanocytes [36]. Proteasomal degradation of β-catenin occurs through GSK3β-mediated phosphorylation at the serine-33, serine-37, serine-45, and threonine-41 residues of β-catenin. Studies have shown that phosphorylation at the Tyr-216 residue of GSK3β significantly enhances the enzymatic activity of GSK3β, whereas the phosphorylation at the 9th serine residue of GSK3β inactivates it. β-catenin has previously been reported to increase MITF expression. Therefore, strategic inactivation of β-catenin resulted in a decline in MITF levels. Thymoquinone dose-dependently reduced the expression of MITF and tyrosinase that was accompanied by decreased tyrosinase activity in B16F10 cells. Pre-treatment with LiCl resulted in an increase in the levels of p-GSK3β that led to the blockade of β-catenin degradation and increased the expression and activity of tyrosinase [36].
The generation of hybrids between conventional chemotherapeutic and bioactive natural products is an innovative approach to obtain effective anticancer compounds [37]. A hybrid of thymoquinone and 5-fluorouracil not only reduced β-catenin levels but also suppressed transcriptional activity of β-catenin in colorectal cancer cell lines. HCT116 control cells developed highly vascularized tumor masses, whereas hybrid-treated-tumor xenografts were significantly smaller in size. Interesting, there were signs of cellular proliferation in the xenografts combinatorically treated with thymoquinone and 5-fluorouracil, whereas hybrid-treated tumors did not show any sign of mitotic activity [37].
Intraperitoneally injected thymoquinone reduced the size and number of aberrant crypt foci and tumor multiplicities in a chemical-induced model of colorectal cancer [38]. Thymoquinone reduced polyp growth and selectively induced apoptosis. High doses of thymoquinone led to the translocation of β-catenin to the membrane and a reduction in the large polyps of APCMin mice [38].
It has been shown that the phosphorylation of GSK3β at serine-9 induced inactivation. Therefore, thymoquinone reduced p-GSK3β, β-catenin, and MMP2/MMP9 in Eca109 cells [39].
4. Regulation of UHRF1 by Thymoquinone
The ubiquitin-like protein containing PHD and RING fingers domains-1 (UHRF1) is a multi-domain-containing protein. UHRF1 maintains DNA methylation through the recruitment of DNA methyltransferase-1 to the replication forks in the S-phases of the cells.
Thymoquinone induced an auto-ubiquitination of UHRF1 through its RING domain [40]. The protein p73 is a functional and structural homologue of p53. P73 induced apoptosis in cancer cells. However, UHRF1 epigenetically inactivated p73 in Jurkat cells. However, thymoquinone-mediated auto-ubiquitination of UHRF1 caused a sharp increase in the expression of p73 in Jurkat cells [40].
UHRF1 binds to the inverted CCAAT domain in the promoter region of TXNIP and inhibits its expression via CpG methylation [41]. UHRF1 knockdown inhibited UHRF1 binding to the promoter of TXNIP and enhanced TXNIP expression through promoter demethylation in HeLa cells. Levels of ubiquitin-specific protease-7 (USP7) were noted to be enhanced in HPV16 E6/E7-overexpressing cells. USP7 efficiently stabilized UHRF1 and epigenetically inactivated TXNIP [41].
Likewise, HPV E6/E7 caused a marked increase in the levels of USP7 and UHRF1. UHRF1 effectively inactivated gelsolin in HeLa cells, whereas thymoquinone induced the expression of gelsolin and promoted apoptotic death in cancer cells [42].
5. PI3K/AKT/mTOR
The PI3K/AKT/mTOR pathway has long been an attractive target in molecular oncology. Research teams have focused on the pharmacological targeting of key components of this signaling network [43][44][45][46][47].
Thymoquinone dose-dependently reduced the levels of p-AKT (threonine-308), p-AKT (serine-473), p-mTOR1, and p-mTOR2 in gastric cancer cells. Thymoquinone inhibited the colony formation and invasive capacities of gastric cancer cells [48].
Thymoquinone alone and with pre-treatment significantly reduced p-AKT in BxPC-3, AsPC-1, and PANC-1 cells [49]. Thymoquinone pre-treatment markedly impaired gemcitabine-mediated increases in the phosphorylation of mTOR and S6 in pancreatic cancer cells. More importantly, thymoquinone and gemcitabine induced shrinkage of the primary tumors in the orthotopic cancer models of PANC-1 cells. Pre-treatment with thymoquinone led to marked suppression in p-AKT, p-mTOR, and p-S6 in tumor tissues of xenografted mice [49].
AMPK activates autophagy by inhibition of mTOR [50]. The inhibition of mTORC1 increased autophagy, whereas mTORC1 activation caused deactivation of autophagy. Thymoquinone caused an increase in the level of p-AMPK in 786-O and ACHN cells, while levels of p-mTOR and p-S6K were reduced. There was a significant decline in the number of metastatic nodules in the lungs of thymoquinone-treated mice [50].
Thymoquinone effectively inhibited the PI3K/AKT/mTOR pathway independently and in combination with 5-fluorouracil and active vitamin D3 in colorectal cancer cells [51].
6. NF-κb Activity in Carcinogenesis: Paradoxical Roles of Thymoquinone
Learning more about the complicated mechanisms of NF-κB regulation can be advantageous in the design and development of better therapeutic approaches to target versatile transcriptional factor in different types of cancers. NF-κB functions are controlled tightly by several regulatory proteins, and a disruption of this process has been associated with carcinogenesis and metastasis [52][53][54][55]. NF-κB provides a mechanistic linkage between cancer and inflammation and is a main regulator controlling the capability of malignant cells to trigger pro-survival signaling and resist apoptotic cell death.
Thymoquinone stimulated the expression levels of miR-603 in MDA-MB-436 and MDA-MB-231 cancer cells. NF-κB transcriptionally repressed miR-603. Importantly, miR-603 directly targeted eEF-2K and inhibited the proliferation and invasive capacities of breast cancer cells. Intravenously administered thymoquinone-loaded liposomal nanoparticles impaired tumor growth in mice orthotopically implanted with MDA-MB-231 and MDA-MB-436 cancer cells [56].
Thymoquinone and bortezomib combinatorially induced tumor shrinkage in mice subcutaneously implanted with U266 cells. NF-κB activity was significantly reduced in the tumor tissues of mice xenografted with multiple myeloma cells [57].
There was a significant decrease in the ratio of p-NF-κB/NF-κB in tumor xenografts from mice combinatorially treated with thymoquinone and cisplatin [58]
Cancer-promoting role of Thymoquinone: Prolonged thymoquinone treatment results in an increase in NF-κB reporter activities and induced a two-fold rise in volume of the ascites [61].
ID8-NGL mouse ovarian cancer cells stably expressing NF-κB have been investigated to analyze the cancer chemopreventive effects of thymoquinone. ID8-NGL cells were intraperitoneally injected into a C57BL/6 rodent model. Prolonged treatment of thymoquinone (30 days) resulted in an increase in the activity of NF-κB in tumors. Moreover, prolonged treatment of thymoquinone induced pro-tumor M2-like macrophages within the tumor microenvironments. Thymoquinone triggered an increase in the infiltration of macrophages. Thymoquinone caused an overall increase in the expression of IL-1β and TNFα in macrophages and VEGF in ascites fluid. M2-like macrophages produced high concentrations of signaling molecules such as TNFα, which increased the activity of NF-κB in the tumor tissues, consequently resulting in drug resistance in a sub-population of cancer cells [62].
This entry is adapted from the peer-reviewed paper 10.3390/ijms23116311
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Puisieux, A.; Brabletz, T.; Caramel, J. Oncogenic roles of EMT-inducing transcription factors. Nat. Cell Biol. 2014, 16, 488–494.
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84.
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292.
- Hamann, L.G. Synthetic strategy: Natural products on demand. Nat. Chem. 2014, 6, 460–461.
- Mann, J. Natural products in cancer chemotherapy: Past, present and future. Nat. Rev. Cancer 2002, 2, 143–148.
- Hann, M.M.; Keserü, G.M. Finding the sweet spot: The role of nature and nurture in medicinal chemistry. Nat. Rev. Drug Discov. 2012, 11, 355–365.
- Keserü, G.M.; Makara, G.M. The influence of lead discovery strategies on the properties of drug candidates. Nat. Rev. Drug Discov. 2009, 8, 203–212.
- Lombardino, J.G.; Lowe, J.A., III. The role of the medicinal chemist in drug discovery—Then and now. Nat. Rev. Drug Discov. 2004, 3, 853–862.
- Harphoush, S.; Wu, G.; Qiuli, G.; Zaitoun, M.; Ghanem, M.; Shi, Y.; Le, G. Thymoquinone ameliorates obesity-induced metabolic dysfunction, improves reproductive efficiency exhibiting a dose-organ relationship. Syst. Biol. Reprod. Med. 2019, 65, 367–382.
- Karandrea, S.; Yin, H.; Liang, X.; Slitt, A.L.; Heart, E.A. Thymoquinone ameliorates diabetic phenotype in diet-induced obesity mice via activation of SIRT-1-dependent pathways. PLoS ONE 2017, 12, e0185374.
- Badr, G.; Mahmoud, M.H.; Farhat, K.; Waly, H.; Al-Abdin, O.Z.; Rabah, D.M. Maternal supplementation of diabetic mice with thymoquinone protects their offspring from abnormal obesity and diabetes by modulating their lipid profile and free radical production and restoring lymphocyte proliferation via PI3K/AKT signaling. Lipids Health Dis. 2013, 12, 37.
- Alshahrani, S.; Anwer, T.; Alam, M.F.; Ahmed, R.A.; Khan, G.; Sivakumar, S.M.; Shoaib, A.; Alam, P.; Azam, F. Effect of thymoquinone on high fat diet and STZ-induced experimental type 2 diabetes: A mechanistic insight by in vivo and in silico studies. J. Food Biochem. 2021, e13807.
- El-Shemi, A.G.; Kensara, O.A.; Alsaegh, A.; Mukhtar, M.H. Pharmacotherapy with thymoquinone improved pancreatic β-cell integrity and functional activity, enhanced islets revascularization, and alleviated metabolic and hepato-renal disturbances in streptozotocin-induced diabetes in rats. Pharmacology 2018, 101, 9–21.
- Khan, M.A.; Younus, H. Thymoquinone shows the diverse therapeutic actions by modulating multiple cell signaling pathways: Single drug for multiple targets. Curr. Pharm. Biotechnol. 2018, 19, 934–945.
- Majdalawieh, A.F.; Fayyad, M.W.; Nasrallah, G.K. Anti-cancer properties and mechanisms of action of thymoquinone, the major active ingredient of Nigella sativa. Crit. Rev. Food Sci. Nutr. 2017, 57, 3911–3928.
- Schneider-Stock, R.; Fakhoury, I.H.; Zaki, A.M.; El-Baba, C.O.; Gali-Muhtasib, H.U. Thymoquinone: Fifty years of success in the battle against cancer models. Drug Discov. Today 2014, 19, 18–30.
- Banerjee, S.; Padhye, S.; Azmi, A.; Wang, Z.; Philip, P.A.; Kucuk, O.; Sarkar, F.H.; Mohammad, R.M. Review on molecular and therapeutic potential of thymoquinone in cancer. Nutr. Cancer 2010, 62, 938–946.
- Gali-Muhtasib, H.; Roessner, A.; Schneider-Stock, R. Thymoquinone: A promising anti-cancer drug from natural sources. Int. J. Biochem. Cell Biol. 2006, 38, 1249–1253.
- Thomas, S.J.; Snowden, J.A.; Zeidler, M.P.; Danson, S.J. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br. J. Cancer 2015, 113, 365–371.
- Recio, C.; Guerra, B.; Guerra-Rodríguez, M.; Aranda-Tavío, H.; Martín-Rodríguez, P.; de Mirecki-Garrido, M.; Brito-Casillas, Y.; García-Castellano, J.M.; Estévez-Braun, A.; Fernández-Pérez, L. Signal transducer and activator of transcription (STAT)-5: An opportunity for drug development in oncohematology. Oncogene 2019, 38, 4657–4668.
- Miklossy, G.; Hilliard, T.S.; Turkson, J. Therapeutic modulators of STAT signalling for human diseases. Nat. Rev. Drug Discov. 2013, 12, 611–629.
- Raut, P.K.; Lee, H.S.; Joo, S.H.; Chun, K.S. Thymoquinone induces oxidative stress-mediated apoptosis through downregulation of Jak2/STAT3 signaling pathway in human melanoma cells. Food Chem. Toxicol. 2021, 157, 112604.
- Hatiboglu, M.A.; Kocyigit, A.; Guler, E.M.; Akdur, K.; Khan, I.; Nalli, A.; Karatas, E.; Tuzgen, S. Thymoquinone enhances the effect of gamma knife in B16-F10 melanoma through inhibition of phosphorylated STAT3. World Neurosurg. 2019, 128, e570–e581.
- Hatiboglu, M.A.; Kocyigit, A.; Guler, E.M.; Akdur, K.; Nalli, A.; Karatas, E.; Tuzgen, S. Thymoquinone induces apoptosis in B16-F10 melanoma cell through inhibition of p-STAT3 and inhibits tumor growth in a murine intracerebral melanoma model. World Neurosurg. 2018, 114, e182–e190.
- Chae, I.G.; Song, N.Y.; Kim, D.H.; Lee, M.Y.; Park, J.M.; Chun, K.S. Thymoquinone induces apoptosis of human renal carcinoma Caki-1 cells by inhibiting JAK2/STAT3 through pro-oxidant effect. Food Chem. Toxicol. 2020, 139, 111253.
- Park, J.E.; Kim, D.H.; Ha, E.; Choi, S.M.; Choi, J.S.; Chun, K.S.; Joo, S.H. Thymoquinone induces apoptosis of human epidermoid carcinoma A431 cells through ROS-mediated suppression of STAT3. Chem. Biol. Interact. 2019, 312, 108799.
- Zhu, W.Q.; Wang, J.; Guo, X.F.; Liu, Z.; Dong, W.G. Thymoquinone inhibits proliferation in gastric cancer via the STAT3 pathway in vivo and in vitro. World J. Gastroenterol. 2016, 22, 4149–4159.
- Hu, X.; Ma, J.; Vikash, V.; Li, J.; Wu, D.; Liu, Y.; Zhang, J.; Dong, W. Thymoquinone augments cisplatin-induced apoptosis on esophageal carcinoma through mitigating the activation of JAK2/STAT3 pathway. Dig. Dis. Sci. 2018, 63, 126–134.
- Al-Rawashde, F.A.; Johan, M.F.; Taib, W.R.W.; Ismail, I.; Johari, S.A.T.T.; Almajali, B.; Al-Wajeeh, A.S.; Nazari Vishkaei, M.; Al-Jamal, H.A.N. Thymoquinone inhibits growth of acute myeloid leukemia cells through reversal SHP-1 and SOCS-3 hypermethylation: In vitro and in silico evaluation. Pharmaceuticals 2021, 14, 1287.
- Korinek, V.; Barker, N.; Morin, P.J.; van Wichen, D.; de Weger, R.; Kinzler, K.W.; Vogelstein, B.; Clevers, H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science 1997, 275, 1784–1787.
- Morin, P.J.; Sparks, A.B.; Korinek, V.; Barker, N.; Clevers, H.; Vogelstein, B.; Kinzler, K.W. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 1997, 275, 1787–1790.
- Suzuki, H.; Watkins, D.N.; Jair, K.W.; Schuebel, K.E.; Markowitz, S.D.; Chen, W.D.; Pretlow, T.P.; Yang, B.; Akiyama, Y.; Van Engeland, M.; et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat. Genet. 2004, 36, 417–422.
- Behrens, J.; Jerchow, B.A.; Würtele, M.; Grimm, J.; Asbrand, C.; Wirtz, R.; Kühl, M.; Wedlich, D.; Birchmeier, W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science 1998, 280, 596–599.
- Zhang, M.; Du, H.; Wang, L.; Yue, Y.; Zhang, P.; Huang, Z.; Lv, W.; Ma, J.; Shao, Q.; Ma, M.; et al. Thymoquinone suppresses invasion and metastasis in bladder cancer cells by reversing EMT through the Wnt/β-catenin signaling pathway. Chem. Biol. Interact. 2020, 320, 109022.
- Jeong, H.; Yu, S.M.; Kim, S.J. Inhibitory effects on melanogenesis by thymoquinone are mediated through the β-catenin pathway in B16F10 mouse melanoma cells. Int. J. Oncol. 2020, 56, 379–389.
- Ndreshkjana, B.; Çapci, A.; Klein, V.; Chanvorachote, P.; Muenzner, J.K.; Huebner, K.; Steinmann, S.; Erlenbach-Wuensch, K.; Geppert, C.I.; Agaimy, A.; et al. Combination of 5-fluorouracil and thymoquinone targets stem cell gene signature in colorectal cancer cells. Cell Death Dis. 2019, 10, 379.
- Lang, M.; Borgmann, M.; Oberhuber, G.; Evstatiev, R.; Jimenez, K.; Dammann, K.W.; Jambrich, M.; Khare, V.; Campregher, C.; Ristl, R.; et al. Thymoquinone attenuates tumor growth in ApcMin mice by interference with Wnt-signaling. Mol. Cancer 2013, 12, 41.
- Ma, J.; Zhang, Y.; Deng, H.; Liu, Y.; Lei, X.; He, P.; Dong, W. Thymoquinone inhibits the proliferation and invasion of esophageal cancer cells by disrupting the AKT/GSK-3β/Wnt signaling pathway via PTEN upregulation. Phytother. Res. 2020, 34, 3388–3399.
- Ibrahim, A.; Alhosin, M.; Papin, C.; Ouararhni, K.; Omran, Z.; Zamzami, M.A.; Al-Malki, A.L.; Choudhry, H.; Mély, Y.; Hamiche, A.; et al. Thymoquinone challenges UHRF1 to commit auto-ubiquitination: A key event for apoptosis induction in cancer cells. Oncotarget 2018, 9, 28599–28611.
- Kim, M.J.; Lee, H.J.; Choi, M.Y.; Kang, S.S.; Kim, Y.S.; Shin, J.K.; Choi, W.S. UHRF1 induces methylation of the TXNIP promoter and down-regulates gene expression in cervical cancer. Mol. Cells 2021, 44, 146–159.
- Lee, H.J.; Kim, M.J.; Kim, Y.S.; Choi, M.Y.; Cho, G.J.; Choi, W.S. UHRF1 silences gelsolin to inhibit cell death in early stage cervical cancer. Biochem. Biophys. Res. Commun. 2020, 526, 1061–1068.
- Hennessy, B.T.; Smith, D.L.; Ram, P.T.; Lu, Y.; Mills, G.B. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005, 4, 988–1004.
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644.
- Janku, F.; Yap, T.A.; Meric-Bernstam, F. Targeting the PI3K pathway in cancer: Are we making headway? Nat. Rev. Clin. Oncol. 2018, 15, 273–291.
- Janku, F.; Hong, D.S.; Fu, S.; Piha-Paul, S.A.; Naing, A.; Falchook, G.S.; Tsimberidou, A.M.; Stepanek, V.M.; Moulder, S.L.; Lee, J.J.; et al. Assessing PIK3CA and PTEN in early-phase trials with PI3K/AKT/mTOR inhibitors. Cell Rep. 2014, 6, 377–387.
- Ihle, N.T.; Lemos, R., Jr.; Wipf, P.; Yacoub, A.; Mitchell, C.; Siwak, D.; Mills, G.B.; Dent, P.; Kirkpatrick, D.L.; Powis, G. Mutations in the phosphatidylinositol-3-kinase pathway predict for antitumor activity of the inhibitor PX-866 whereas oncogenic Ras is a dominant predictor for resistance. Cancer Res. 2009, 69, 143–150.
- Feng, L.M.; Wang, X.F.; Huang, Q.X. Thymoquinone induces cytotoxicity and reprogramming of EMT in gastric cancer cells by targeting PI3K/Akt/mTOR pathway. J. Biosci. 2017, 42, 547–554.
- Mu, G.G.; Zhang, L.L.; Li, H.Y.; Liao, Y.; Yu, H.G. Thymoquinone pretreatment overcomes the insensitivity and potentiates the antitumor effect of gemcitabine through abrogation of Notch1, PI3K/Akt/mTOR regulated signaling pathways in pancreatic cancer. Dig. Dis. Sci. 2015, 60, 1067–1080.
- Zhang, Y.; Fan, Y.; Huang, S.; Wang, G.; Han, R.; Lei, F.; Luo, A.; Jing, X.; Zhao, L.; Gu, S.; et al. Thymoquinone inhibits the metastasis of renal cell cancer cells by inducing autophagy via AMPK/mTOR signaling pathway. Cancer Sci. 2018, 109, 3865–3873.
- Idris, S.; Refaat, B.; Almaimani, R.A.; Ahmed, H.G.; Ahmad, J.; Alhadrami, M.; El-Readi, M.Z.; Elzubier, M.E.; Alaufi, H.A.A.; Al-Amin, B.; et al. Enhanced in vitro tumoricidal effects of 5-Fluorouracil, thymoquinone, and active vitamin D3 triple therapy against colon cancer cells by attenuating the PI3K/AKT/mTOR pathway. Life Sci. 2022, 296, 120442.
- Pikarsky, E.; Porat, R.M.; Stein, I.; Abramovitch, R.; Amit, S.; Kasem, S.; Gutkovich-Pyest, E.; Urieli-Shoval, S.; Galun, E.; Ben-Neriah, Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004, 431, 461–466.
- Greten, F.R.; Eckmann, L.; Greten, T.F.; Park, J.M.; Li, Z.W.; Egan, L.J.; Kagnoff, M.F.; Karin, M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004, 118, 285–296.
- Senftleben, U.; Cao, Y.; Xiao, G.; Greten, F.R.; Krähn, G.; Bonizzi, G.; Chen, Y.; Hu, Y.; Fong, A.; Sun, S.C.; et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science 2001, 293, 1495–1499.
- Erez, N.; Truitt, M.; Olson, P.; Arron, S.T.; Hanahan, D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell 2010, 17, 135–147.
- Kabil, N.; Bayraktar, R.; Kahraman, N.; Mokhlis, H.A.; Calin, G.A.; Lopez-Berestein, G.; Ozpolat, B. Thymoquinone inhibits cell proliferation, migration, and invasion by regulating the elongation factor 2 kinase (eEF-2K) signaling axis in triple-negative breast cancer. Breast Cancer Res. Treat. 2018, 171, 593–605.
- Siveen, K.S.; Mustafa, N.; Li, F.; Kannaiyan, R.; Ahn, K.S.; Kumar, A.P.; Chng, W.J.; Sethi, G. Thymoquinone overcomes chemoresistance and enhances the anticancer effects of bortezomib through abrogation of NF-κB regulated gene products in multiple myeloma xenograft mouse model. Oncotarget 2014, 5, 634–648.
- Jafri, S.H.; Glass, J.; Shi, R.; Zhang, S.; Prince, M.; Kleiner-Hancock, H. Thymoquinone and cisplatin as a therapeutic combination in lung cancer: In vitro and in vivo. J. Exp. Clin. Cancer Res. 2010, 29, 87.
- Chen, M.C.; Lee, N.H.; Hsu, H.H.; Ho, T.J.; Tu, C.C.; Chen, R.J.; Lin, Y.M.; Viswanadha, V.P.; Kuo, W.W.; Huang, C.Y. Inhibition of NF-κB and metastasis in irinotecan (CPT-11)-resistant LoVo colon cancer cells by thymoquinone via JNK and p38. Environ. Toxicol. 2017, 32, 669–678.
- Alshyarba, M.; Otifi, H.; Al Fayi, M.; ADera, A.; Rajagopalan, P. Thymoquinone inhibits IL-7-induced tumor progression and metastatic invasion in prostate cancer cells by attenuating matrix metalloproteinase activity and Akt/NF-κB signaling. Biotechnol. Appl. Biochem. 2021, 68, 1403–1411.
- Wilson, A.J.; Saskowski, J.; Barham, W.; Yull, F.; Khabele, D. Thymoquinone enhances cisplatin-response through direct tumor effects in a syngeneic mouse model of ovarian cancer. J. Ovarian Res. 2015, 8, 46.
- Wilson, A.J.; Saskowski, J.; Barham, W.; Khabele, D.; Yull, F. Microenvironmental effects limit efficacy of thymoquinone treatment in a mouse model of ovarian cancer. Mol. Cancer 2015, 14, 192.
This entry is offline, you can click here to edit this entry!
