Moringa oleifera Lam. (Moringaceae) is one of the most essential medicinal plants primarily found in the rainforest area and forest ecosystem, but is now well-adapted in an organized cultivation system. Moringa oleifera (M. oleifera) is well-known as Drumstick tree, Moringa kai, color, Marengo, Moringe, mulangay, Sahjan, and Sajna, which are its native names commonly used. It has nourishing, beneficial, and preventive effects when taken as food and has an extensive scope of high restorative properties with huge dietary benefits. Different parts of the M. oleifera plants, such as leaves, flowers, fruits, seeds, and roots, contain a significant amount of protein, ß-carotene, amino acids, important minerals, and various phenolic compounds. Because of its multifarious health benefits for its therapeutic value, it is considered an essential plant. The plant is found to be blessed with several medicinal characteristics such as antitumor, anti-inflammatory, antiulcer, antipyretic, antiepileptic, antispasmodic, diuretic, antihypertensive, antidiabetic, cholesterol-level down, cell reinforcement, and hepatoprotective. Moreover, it is used traditionally in the local curative system against cardiac problems, and the antifungal properties are efficiently utilized for the treatment of a wide range of ailments.
- Moringa oleifera
- medicinal and pharmaceuticals
- nutritional
- economic importance
- therapeutics
- horticultural crops
1. Introduction
2. Industrial Applications
2.1. Treatment of Water
2.2. Great Fodder for Cattle
2.3. Bio-Gas
2.4. Standard Plate Count (SPC) Method
2.5. Most Probable Number (MPN)
2.6. Other Industrial Uses
3. Economic Potential
4. Toxicity Levels
5. Medicinal Properties and Biomedical Applications
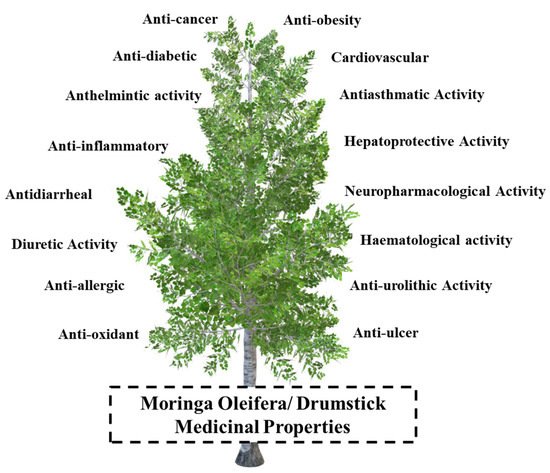
5.1. Analgesic, Anti-Inflammatory, and Antipyretic Activities
5.2. Neuropharmacological Activity
5.3. Anticancerous Activity
5.4. Antioxidant Activity
5.5. Hepatoprotective Activity
5.6. Anti-Ulcer and Gastroprotective Properties
5.7. Cardiovascular Activity
5.8. Antiobesity Activity
5.9. Antiasthmatic Activity
5.10. Hematological Activity
5.11. Antidiabetic Activity
5.12. Anti-Urolithic Activity
5.13. Diuretic Activity
5.14. Anti-Allergic Activity
5.15. Anthelmintic Activity
5.16. Antidiarrheal Activity
5.17. Diabetes and Diverse Effects
This entry is adapted from the peer-reviewed paper 10.3390/horticulturae8060492
References
- Ramachandran, C.; Peter, K.V.; Gopalakrishnan, P.K. Drumstick (Moringa oleifera): A multipurpose Indian vegetable. Econ. Bot. 1980, 34, 276–283.
- Nadkarni, K.; Nadkarni, A. Indian Materia Medica; Popular Prakashan Pvt. Ltd.: Mumbai, India, 1976; Volume 1, p. 1799.
- Kardam, A.; Raj, K.R.; Arora, J.K.; Srivastava, M.M.; Srivastava, S. Artificial Neural Network Modeling for Sorption of Cadmium from Aqueous System by Shelled Moringa Oleifera Seed Powder as an Agricultural Waste. J. Water Resour. Prot. 2010, 2, 339–344.
- Anwar, F.; Bhanger, M.I. Analytical Characterization of Moringa oleifera Seed Oil Grown in Temperate Regions of Pakistan. J. Agric. Food Chem. 2003, 51, 6558–6563.
- D’souza, J.; Kulkarni, A. Comparative Studies on Nutritive Values of Tender Foliage of Seedlings, and Mature Plants of Moringa oleifera (Lamk). Indian J. Nutr. Dietetics 1990, 27, 205–212.
- Somali, M.A.; Bajneid, M.A.; Al-Fhaimani, S.S. Chemical composition and characteristics of Moringa peregrina seeds and seeds oil. J. Am. Oil Chem. Soc. 1984, 61, 85–86.
- Oinam, N.; Urooj, A.; Phillips, P.P.; Niranjan, N.P. Effect of dietary lipids and drumstick leaves (Moringa oleifera) on lipid profile & antioxidant parameters in rats. Food Nutr. Sci. 2012, 3, 141–145.
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756.
- Siddhuraju, P.; Becker, K. Antioxidant Properties of Various Solvent Extracts of Total Phenolic Constituents from Three Different Agroclimatic Origins of Drumstick Tree (Moringa oleifera Lam.) Leaves. J. Agric. Food Chem. 2003, 51, 2144–2155.
- Saini, R.K.; Sivanesan, I.; Keum, Y.-S. Phytochemicals of Moringa oleifera: A review of their nutritional, therapeutic and industrial significance. 3 Biotech 2016, 6, 203.
- Patel, S.; Thakur, A.S.; Chandy, A.; Manigauha, A. Moringa oleifera: A review of there medicinal and economical importance to the health and nation. Drug Invent. Today 2010, 2, 339–342.
- Kirtikar, K.; Basu, B. New Cannaught Place; M/s Bishen Singh Mahendrapal Singh: Dehradun, India, 1975.
- Lea, M. Bioremediation of Turbid Surface Water Using Seed Extract from the Moringa oleifera Lam. (Drumstick) Tree. Curr. Protoc. Microbiol. 2014, 33, 1–8.
- Tahiliani, P.; Kar, A. Role of Moringa oleifera leaf extract in the regulation of thyroid hormone status in adult male and female rats. Pharmacol. Res. 2000, 41, 319–323.
- Morton, J.F. The horseradish tree, Moringa pterygosperma (Moringaceae)—A boon to arid lands? Econ. Bot. 1991, 45, 318–333.
- Jahn, S.; Musnad, H.A.; Burgstaller, H. The tree that purifies water: Cultivating multipurpose Moringaceae in the Sudan. Unasylva 1986, 38, 23–28.
- Radovich, T. Farm and Forestry Production and Marketing Profile for Moringa (Moringa oleifera); Permanent Agriculture Resources (PAR): Holualoa, HI, USA, 2011.
- Trigo, C.; Castelló, M.L.; Ortolá, M.D.; García-Mares, F.J.; Soriano, M.D. Moringa oleifera: An Unknown Crop in Developed Countries with Great Potential for Industry and Adapted to Climate Change. Foods 2020, 10, 31.
- Rajangam, J.; Azahakia Manavalan, R.S.; Thangaraj, T.; Vijayakumar, A.; Muthukrishan, N. Status of Production and Utilization of Moringa in Southern India. Development Potential for Moringa Product. Dar Es Salaam, Tanzania. 2001. Available online: http://www.moringanews.org/actes/rajangam_en.doc (accessed on 10 January 2022).
- Baptista, A.T.A.; Silva, M.O.; Gomes, R.G.; Bergamasco, R.; Vieira, M.F.; Vieira, A.M.S. Protein fractionation of seeds of Moringa oleifera lam and its application in superficial water treatment. Sep. Purif. Technol. 2017, 180, 114–124.
- James, A.; Zikankuba, V. Moringa oleifera a potential tree for nutrition security in sub-Sahara Africa. Am. J. Res. Commun. 2017, 5, 1–14.
- Gautam, R.K.; Sankaran, M.; Zamir Ahmed, S.K.; AI Sunder, J.; Ram, N.; Dam Roy, S. Custodian Farmers and Communities of Biodiversity Conservation and Utilization in Andaman & Nicobar Islands, India. ICAR-CIARI, Port Blair; 2014. Available online: http://krishi.icar.gov.in/jspui/handle/123456789/20006 (accessed on 11 April 2022).
- Palada, M.C. Moringa (Moringa oleifera Lam.): A Versatile Tree Crop with Horticultural Potential in the Subtropical United States. HortScience 1996, 31, 794–797.
- Godino, M.; Arias, C.; Izquierdo, M. Interés forestal de la Moringa Oleifera y Posibles Zonas de Implantación en España. In 6° Congreso Forestal Español: “Montes: Servicios y Desarrollo Rural”; Sociedad Española de Ciencias Forestales: Barcelona, Spain, 2013.
- Liu, Y.; Wang, X.-Y.; Wei, X.-M.; Gao, Z.-T.; Han, J.-P. Values, properties and utility of different parts of Moringa oleifera: An overview. Chin. Herb. Med. 2018, 10, 371–378.
- Senthilkumar, A.; Karuvantevida, N.; Rastrelli, L.; Kurup, S.S.; Cheruth, A.J. Traditional Uses, Pharmacological Efficacy, and Phytochemistry of Moringa peregrina (Forssk.) Fiori. —A Review. Front. Pharmacol. 2018, 9, 465.
- Bhattacharya, A.; Agrawal, D.; Sahu, P.K.; Kumar, S.; Mishra, S.S.; Patnaik, S. Analgesic effect of ethanolic leaf extract of Moringa oleifera on albino mice. Indian J. Pain 2014, 28, 89.
- Bosch, C. Moringa stenopetala (Baker f.) Cufod. PROTA 2004, 2, 395–397.
- George, T.T.; Oyenihi, A.B.; Rautenbach, F.; Obilana, A.O. Characterization of Moringa oleifera Leaf Powder Extract Encapsulated in Maltodextrin and/or Gum Arabic Coatings. Foods 2021, 10, 3044.
- Chawla, S.; Saxena, A.; Seshadri, S. In-vitro availability of iron in various green leafy vegetables. J. Sci. Food Agric. 1988, 46, 125–127.
- Dogra, P.; Singh, B.; Tandon, S. Vitamin C content in moringa pod vegetable. Curr. Sci. 1975, 44, 31.
- Azam, M.M.; Waris, A.; Nahar, N. Prospects and potential of fatty acid methyl esters of some non-traditional seed oils for use as biodiesel in India. Biomass Bioenergy 2005, 29, 293–302.
- Nadkarni, K.M. Indian materia medica: With Ayurvedic, Unani-Tibbi, Siddha, allopathic, homeopathic, naturopathic & home remedies, appendices & indexes. 1. In Indian Materia Medica; Popular Prakashan: Mumbai, India, 1996; Volume 1.
- Gilani, A.; Janbaz, K.H.; Shah, B.H. 85 Quercetin exhibits hepatoprotective activity in rats. Biochem. Soc. Trans. 1997, 25, S619.
- Woomer, P.L. Most Probable Number Counts. In Methods of Soil Analysis: Part 2 Microbiological and Biochemical Properties; Wiley Online Library: Hoboken, NJ, USA, 2018; pp. 59–79.
- Adedapo, A.; Falayi, F.; Oyagbemi, A. Evaluation of the analgesic, anti-inflammatory, anti-oxidant, phytochemical and toxicological properties of the methanolic leaf extract of commercially processed Moringa oleifera in some laboratory animals. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 491–499.
- Ferrao, A.M.B.C.; Ferrao, M.J.E. Ácidos gordos em óleo de Moringueiro (Moringa oleifera Lam.). Agron. Angolana 1970, 8, 3–16.
- Fuglie, L.J. The Miracle Tree: Moringa Oleifera. Natural Nutrition for the Tropics; Food and Agriculture Organization of the United Nations: Rome, Italy, 1999.
- Jarald, E.E.; Sumati, S.; Edwin, S.; Ahmad, S.; Patni, S.; Daud, A. Characterization of Moringa oleifera Lam. gum to establish it as a pharmaceutical excipient. Indian J. Pharm. Educ. Res. 2012, 46, 211–216.
- Verma, S.C.; Banerji, R.; Misra, G.; Nigam, S.K. Nutritional value of Moringa. Curr. Sci. 1976, 45, 769–770.
- Sutar, N.G.; Bonde, C.G.; Patil, V.V.; Narkhede, S.B.; Patil, A.P.; Kakade, R.T. Analgesic activity of seeds of Moringa oleifera Lam. Int. J. Green Pharm. 2008, 2.
- Manaheji, H. Analgesic effects of methanolic extracts of the leaf or root of Moringa oleifera on complete Freund’s adjuvant-induced arthritis in rats. J. Chin. Integr. Med. 2011, 9, 216–222.
- Upadhye, K.; Rangari, V.; Mathur, V. Antimigraine activity study of Moringa oleifera leaf juice. Int. J. Green Pharm. 2012, 6, 204.
- Velaga, V.S.A.R.; Suryadevara, N.; Chee, L.; Ismail, N.E. Phytochemical analysis and immuno-modulatory effect of Moringa oleifera flowers. Int. J. Pharm. Pharmaceut. 2017, 9, 24–28.
- Ndabigengesere, A.; Narasiah, K.S. Quality of water treated by coagulation using Moringa oleifera seeds. Water Res. 1998, 32, 781–791.
- Ezeamuzie, I.C.; Ambakederemo, A.W.; Shode, F.O.; Ekwebelem, S.C. Antiinflammatory Effects of Moringa oleifera Root Extract. Int. J. Pharmacogn. 1996, 34, 207–212.
- Kinase, J. Moringa tea blocks acute lung inflammation induced by swine confinement dust through a mechanism involving TNF-α expression, c-Jun N-terminal kinase activation and neutrophil regulation. Am. J. Immunol. 2014, 10, 73–87.
- Rani, N.Z.A.; Husain, K.; Kumolosasi, E. Moringa Genus: A Review of Phytochemistry and Pharmacology. Front. Pharmacol. 2018, 9, 108.
- Gopalakrishnan, L.; Doriya, K.; Kumar, D.S. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci. Hum. Wellness 2016, 5, 49–56.
- Ganguly, R.; Hazra, R.; Ray, K.; Guha, D. Effect of Moringa oleifera in experimental model of Alzheimer’s disease: Role of antioxidants. Ann. Neurosci. 2010, 12, 33–36.
- Kou, X.; Li, B.; Olayanju, J.B.; Drake, J.M.; Chen, N. Nutraceutical or Pharmacological Potential of Moringa oleifera Lam. Nutrients 2018, 10, 343.
- Mohan, M.; Kaul, N.; Punekar, A.; Girnar, R.; Junnare, P.; Patil, L. Nootropic activity of Moringa oleifera leaves. J. Nat. Remedies 2005, 5, 59–62.
- Akram, M.; Nawaz, A. Effects of medicinal plants on Alzheimer’s disease and memory deficits. Neural Regen. Res. 2017, 12, 660–670.
- More, S.V.; Kumar, H.; Cho, D.-Y.; Yun, Y.-S.; Choi, D.-K. Toxin-Induced Experimental Models of Learning and Memory Impairment. Int. J. Mol. Sci. 2016, 17, 1447.
- Ray, K.; Guha, D. Effect of Moringa oleifera root extract on penicillin-induced epileptic rats. Biog. Amines 2005, 19, 223–231.
- Fathima, S.N.; Vasudevamurthy, S.; Rajkumar, N. A review on phytoextracts with antiepileptic property. J. Pharm. Sci. Res. 2015, 7, 994.
- Kaur, G.; Invally, M.; Sanzagiri, R.; Buttar, H.S. Evaluation of the antidepressant activity of Moringa oleifera alone and in combination with fluoxetine. J. Ayurveda Integr. Med. 2015, 6, 273–279.
- Bhattacharya, A.; Santra, S.; Mahapatra, S.; Sahu, P.K.; Agrawal, D.; Kumar, S. Study of anxiolytic effect of ethanolic extract of drumstick tree leaves on albino mice in a basic neuropharmacology laboratory of a postgraduate teaching institute. J. Health Res. Rev. 2016, 3, 41.
- Islam, M.T.; Martins, N.; Imran, M.; Hameed, A.; Ali, S.W.; Salehi, B.; Ahmad, I.; Hussain, A.; Sharifi-Rad, J. Anxiolytic-like effects of Moringa oleifera in Swiss mice. Cell. Mol. Biol. 2020, 66, 73–77.
- Kumar, S.; Bhattacharya, A.; Tiwari, P.; Sahu, P. A review of the phytochemical and pharmacological characteristics of Moringa oleifera. J. Pharm. Bioallied Sci. 2018, 10, 181–191.
- Sreelatha, S.; Jeyachitra, A.; Padma, P. Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food Chem. Toxicol. 2011, 49, 1270–1275.
- Jung, I.L.; Lee, J.H.; Kang, S.C. A potential oral anticancer drug candidate, Moringa oleifera leaf extract, induces the apoptosis of human hepatocellular carcinoma cells. Oncol. Lett. 2015, 10, 1597–1604.
- Dulay, M.T.; Zaman, N.; Jaramillo, D.; Mody, A.C.; Zare, R.N. Pathogen-Imprinted Organosiloxane Polymers as Selective Biosensors for the Detection of Targeted E. coli. C 2018, 4, 29.
- Tiloke, C.; Phulukdaree, A.; Chuturgoon, A.A. The antiproliferative effect of Moringa oleifera crude aqueous leaf extract on cancerous human alveolar epithelial cells. BMC Complement. Altern. Med. 2013, 13, 226.
- Al-Asmari, A.K.; AlBalawi, S.M.; Athar, T.; Khan, A.Q.; Al-Shahrani, H.; Islam, M. Moringa oleifera as an Anti-Cancer Agent against Breast and Colorectal Cancer Cell Lines. PLoS ONE 2015, 10, e0135814.
- Mojzis, J.; Varinska, L.; Mojzisova, G.; Kostova, I.; Mirossay, L. Antiangiogenic effects of flavonoids and chalcones. Pharmacol. Res. 2008, 57, 259–265.
- Tragulpakseerojn, J.; Yamaguchi, N.; Pamonsinlapatham, P.; Wetwitayaklung, P.; Yoneyama, T.; Ishikawa, N.; Ishibashi, M.; Apirakaramwong, A. Anti-proliferative effect of Moringa oleifera Lam (Moringaceae) leaf extract on human colon cancer HCT116 cell line. Trop. J. Pharm. Res. 2017, 16, 371–378.
- Luqman, S.; Srivastava, S.; Kumar, R.; Maurya, A.K.; Chanda, D. Experimental Assessment of Moringa oleifera Leaf and Fruit for Its Antistress, Antioxidant, and Scavenging Potential Using In Vitro and In Vivo Assays. Evid. Based Complement. Altern. Med. 2012, 2012, 519084.
- Caceres, A.; Saravia, A.; Rizzo, S.; Zabala, L.; De Leon, E.; Nave, F. Pharmacologic properties of Moringa oleifera. 2: Screening for antispasmodic, antiinflammatory and diuretic activity. J. Ethnopharmacol. 1992, 36, 233–237.
- Abd-Rabou, A.A.; Abdalla, A.M.; Ali, N.A.; Zoheir, K.M.A. Moringa oleifera Root Induces Cancer Apoptosis more Effectively than Leave Nanocomposites and Its Free Counterpart. Asian Pac. J. Cancer Prev. 2017, 18, 2141–2149.
- Purwal, L.; Pathak, A.; Jain, U. In vivo anticancer activity of the leaves and fruits of Moringa oleifera on mouse melanoma. Pharmacologyonline 2010, 1, 655–665.
- Greenhalf, W.; Thomas, A. Combination therapy for the treatment of pancreatic cancer. Anti-Cancer Agents Med. Chem. 2011, 11, 418–426.
- Sreelatha, S.; Padma, P.R. Antioxidant Activity and Total Phenolic Content of Moringa oleifera Leaves in Two Stages of Maturity. Mater. Veg. 2009, 64, 303–311.
- Shameer, P.; Mohamed, K.; Sukhen, S. Effect of Moringa oleifera on stress induced brain lipid peroxidation in rats. Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 336–342.
- Kumar, V.; Pandey, N.; Mohan, N.; Singh, R.P. Antibacterial & antioxidant activity of different extract of Moringa oleifera Leaves—An in vitro study. Int. J. Pharm. Sci. Rev. Res. 2012, 12, 89–94.
- Singh, B.N.; Singh, B.R.; Singh, R.L.; Prakash, D.; Dhakarey, R.; Upadhyay, G.; Singh, H.B. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem. Toxicol. 2009, 47, 1109–1116.
- Satish, A.; Reddy, P.V.; Sairam, S.; Ahmed, F.; Urooj, A. Antioxidative Effect and DNA Protecting Property of Moringa oleifera Root Extracts. J. Herbs Spices Med. Plants 2014, 20, 209–220.
- Laoung-On, J.; Jaikang, C.; Saenphet, K.; Sudwan, P. Phytochemical Screening, Antioxidant and Sperm Viability of Nelumbo nucifera Petal Extracts. Plants 2021, 10, 1375.
- Sinha, M.; Das, D.K.; Bhattacharjee, S.; Majumdar, S.; Dey, S. Leaf Extract of Moringa oleifera Prevents Ionizing Radiation-Induced Oxidative Stress in Mice. J. Med. Food 2011, 14, 1167–1172.
- Paliwal, R.; Sharma, V.; Sharma, S. Elucidation of free radical scavenging and antioxidant activity of aqueous and hydro-ethanolic extracts of Moringa oleifera pods. Res. J. Pharm. Technol. 2011, 4, 566–571.
- Atawodi, S.E.; Atawodi, J.C.; Idakwo, G.A.; Pfundstein, B.; Haubner, R.; Wurtele, G.; Bartsch, H.; Owen, R.W. Evaluation of the Polyphenol Content and Antioxidant Properties of Methanol Extracts of the Leaves, Stem, and Root Barks of Moringa oleifera Lam. J. Med. Food 2010, 13, 710–716.
- Patel, R.K.; Patel, M.M.; Kanzariya, N.R.; Vaghela, K.R.; Patel, N.J. In-vitro hepatoprotective activity of Moringa oleifera Lam. leave on isolated rat hepatocytes. Int. J. Pharm. Sci. 2010, 2, 457–463.
- Hamza, A.A. Ameliorative effects of Moringa oleifera Lam seed extract on liver fibrosis in rats. Food Chem. Toxicol. 2010, 48, 345–355.
- Fakurazi, S.; Hairuszah, I.; Nanthini, U. Moringa oleifera Lam prevents acetaminophen induced liver injury through restoration of glutathione level. Food Chem. Toxicol. 2008, 46, 2611–2615.
- Das, N.; Sikder, K.; Ghosh, S.; Fromenty, B.; Dey, S. Moringa oleifera Lam. leaf extract prevents early liver injury and restores antioxidant status in mice fed with high-fat diet. Indian J. Exp. Biol. 2012, 50, 404–412.
- Suganthi, U.R.; Parvatham, R. Efficacy of Moringa oleifera and Aloe vera on aflatoxin Blinduced hepatotoxicityin rats. Res. J. Biotechnol. 2010, 4, 2024.
- Pari, L.; Kumar, N.A. Hepatoprotective activity of Moringa oleifera on antitubercular drug-induced liver damage in rats. J. Med. Food 2002, 5, 171–177.
- Omodanisi, E.; Aboua, Y.G.; Chegou, N.N.; Oguntibeju, O.O. Hepatoprotective, Antihyperlipidemic, and Anti-inflammatory Activity of Moringa oleifera in Diabetic-induced Damage in Male Wistar Rats. Pharmacogn. Res. 2017, 9, 182–187.
- Abd Eldaim, M.A.; Elrasoul, A.S.A.; Elaziz, S.A.A. An aqueous extract from Moringa oleifera leaves ameliorates hepatotoxicity in alloxan-induced diabetic rats. Biochem. Cell Biol. 2017, 95, 524–530.
- Adeyemi, O.S.; Aroge, C.S.; Akanji, M.A. Moringa oleifera-based diet protects against nickel-induced hepatotoxicity in rats. J. Biomed. Res. 2017, 31, 350–357.
- Toppo, R.; Roy, B.K.; Gora, R.H.; Baxla, S.L.; Kumar, P. Hepatoprotective activity of Moringa oleifera against cadmium toxicity in rats. Veter-World 2015, 8, 537–540.
- Ruckmani, K.; Kavimani, S.; Anandan, R.; Jaykar, B. Effect of Moringa oleifera Lam on paracetamol-induced hepatotoxicity. Indian J. Pharm. Sci. 1998, 60, 33.
- Debnath, S.; Guha, D. Role of Moringa oleifera on enterochromaffin cell count and serotonin content of experimental ulcer model. Indian J. Exp. Biol. 2007, 45, 726–731.
- Ndong, M.; Uehara, M.; Katsumata, S.; Sato, S.; Suzuki, K. Preventive Effects of Moringa oleifera (Lam) on Hyperlipidemia and Hepatocyte Ultrastructural Changes in Iron Deficient Rats. Biosci. Biotechnol. Biochem. 2007, 71, 1826–1833.
- Dangi, S.; Jolly, C.; Narayanan, S. Antihypertensive Activity of the Total Alkaloids from the Leaves of Moringa oleifera. Pharm. Biol. 2002, 40, 144–148.
- Randriamboavonjy, J.I.; Loirand, G.; Vaillant, N.; Lauzier, B.; Derbré, S.; Michalet, S.; Pacaud, P.; Tesse, A. Cardiac Protective Effects of Moringa oleifera Seeds in Spontaneous Hypertensive Rats. Am. J. Hypertens. 2016, 29, 873–881.
- Gilani, A.H.; Aftab, K.; Suria, A.; Siddiqui, S.; Salem, R.; Siddiqui, B.S.; Faizi, S. Pharmacological studies on hypotensive and spasmolytic activities of pure compounds from Moringa oleifera. Phytother. Res. 1994, 8, 87–91.
- Nandave, M.; Ojha, S.K.; Joshi, S.; Kumari, S.; Arya, D.S. Moringa oleifera leaf extract prevents isoproterenol-induced myocardial damage in rats: Evidence for an antioxidant, antiperoxidative, and cardioprotective intervention. J. Med. Food 2009, 12, 47–55.
- Barbagallo, I.; Vanella, L.; Distefano, A.; Nicolosi, D.; Maravigna, A.; Lazzarino, G.; Di Rosa, M.; Tibullo, D.; Acquaviva, R.; Volti, G.L. Moringa oleifera Lam. improves lipid metabolism during adipogenic differentiation of human stem cells. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 5223–5232.
- Nahar, S.; Faisal, F.; Iqbal, J.; Rahman, M.M.; Yusuf, A. Antiobesity activity of Moringa oleifera leaves against high fat diet-induced obesity in rats. Int. J. Basic Clin. Pharmacol. 2016, 5, 1263–1268.
- Bais, S.; Singh, G.S.; Sharma, R. Antiobesity and Hypolipidemic Activity of Moringa oleifera Leaves against High Fat Diet-Induced Obesity in Rats. Adv. Biol. 2014, 2014, 162914.
- Pare, D.; Hilou, A.; Ouedraogo, N.; Guenne, S. Ethnobotanical Study of Medicinal Plants Used as Anti-Obesity Remedies in the Nomad and Hunter Communities of Burkina Faso. Medicines 2016, 3, 9.
- Metwally, F.; Rashad, H.; Ahmed, H.H.; Mahmoud, A.; Raouf, E.A.; Abdalla, A.M. Molecular mechanisms of the anti-obesity potential effect of Moringa oleifera in the experimental model. Asian Pac. J. Trop. Biomed. 2017, 7, 214–221.
- Mehta, A.; Agrawal, B. Investigation into the mechanism of action of Moringa oleifera for its anti-asthmatic activity. Orient. Pharm. Exp. Med. 2008, 8, 24–31.
- Goyal, B.R.; Goyal, R.K.; Mehta, A.A. Investigation Into the Mechanism of Anti-Asthmatic Action of Moringa oleifera. J. Diet. Suppl. 2009, 6, 313–327.
- Mahajan, S.G.; Mehta, A.A. Effect of Moringa oleifera Lam. Seed Extract on Ovalbumin-Induced Airway Inflammation in Guinea Pigs. Inhal. Toxicol. 2008, 20, 897–909.
- Suzana, D.; Suyatna, F.D.; Azizahwati; Andrajati, R.; Santi, P.S.; Mun’im, A. Effect of Moringa oleifera Leaves Extract Against Hematology and Blood Biochemical Value of Patients with Iron Deficiency Anemia. J. Young Pharm. 2017, 9, s79–s84.
- Adegbite, O.A.; Omolaso, B.; Seriki, S.A.; Shatima, C. Effects of Moringa oleifera leaves on hematological indices in humans. Ann. Hematol. Oncol. 2016, 3, 1107.
- Archibong, A.N.; Nku, C.O.; Ofem, O.E. Extract of Moringa oleifera attenuates hematological parameters following salt loading. MicroMedicine 2017, 5, 24–30.
- Manohar, V.S.; Jayasree, T.; Kiran Kishore, K.; Mohana Rupa, L.; Dixit, R.; Chandrasekhar, N. Evaluation of hypoglycemic and antihyperglycemic effect of freshly prepared aqueous extract of Moringa oleifera leaves in normal and diabetic rabbits. J. Chem. Pharm. Res. 2012, 4, 249–253.
- Jaiswal, D.; Rai, P.K.; Kumar, A.; Mehta, S.; Watal, G. Effect of Moringa oleifera Lam. leaves aqueous extract therapy on hyperglycemic rats. J. Ethnopharmacol. 2009, 123, 392–396.
- Yassa, H.D.; Tohamy, A.F. Extract of Moringa oleifera leaves ameliorates streptozotocin-induced Diabetes mellitus in adult rats. Acta Histochem. 2014, 116, 844–854.
- Karadi, R.V.; Gadge, N.B.; Alagawadi, K.; Savadi, R.V. Effect of Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. J. Ethnopharmacol. 2006, 105, 306–311.
- Dhongade, H.K.J.; Paikra, B.K.; Gidwani, B. Phytochemistry and Pharmacology of Moringa oleifera Lam. J. Pharmacopunct. 2017, 20, 194–200.
- Villarruel-López, A.; López-de la Mora, D.A.; Vázquez-Paulino, O.D.; Puebla-Mora, A.G.; Torres-Vitela, M.R.; Guerrero-Quiroz, L.A.; Nuño, K. Effect of Moringa oleifera consumption on diabetic rats. BMC Complement. Altern. Med. 2018, 18, 127.
- Choudhury, S.; Sharan, L.; Sinha, M. Antidiarrhoeal potentiality of leaf extracts of Moringa oleifera. Br. J. Appl. Sci. Technol. 2013, 10, 1086–1096.
- Raguindin, P.F.N.; Dans, L.F.; King, J.F. Moringa oleifera as a Galactagogue. Breastfeed. Med. 2014, 9, 323–324.
- Medhi, B.; Khanikor, H.; Lahon, L.; Mohan, P.; Barua, C. Analgesic, Anti-inflammatory and Local Anaesthetic Activity of Moringa pterygosperma in Laboratory Animals. Pharm. Biol. 2003, 41, 248–252.
- Monera, T.G.; Wolfe, A.R.; Maponga, C.C.; Benet, L.Z.; Guglielmo, J. Moringa oleifera leaf extracts inhibit 6beta-hydroxylation of testosterone by CYP3A4. J. Infect. Dev. Ctries. 2008, 2, 379–383.
- Roosdiana, A.; Prasetyawan, S.; Mahdi, C.; Sutrisno, S. Production and Characterization of Bacillus firmus pectinase. J. Pure Appl. Chem. Res. 2013, 2, 35–41.
- Cabeza, M.S.; Baca, F.L.; Puntes, E.M.; Loto, F.; Baigori, M.; Morata, V.I. Selection of psychrotolerant microorganisms producing cold-active pectinases for biotechnological processes at low temperature. Food Technol. Biotechnol. 2011, 49, 187–195.
- Das, B.; Chakraborty, A.; Ghosh, S.; Chakrabarti, K. Studies on the effect of pH and carbon sources on enzyme activities of some pectinolytic bacteria isolated from jute retting water. Turk. J. Biol. 2011, 35, 671–678.
- Namasivayam, E.; Ravindar, J.D.; Mariappan, K.; Jiji, A.; Kumar, M.; Jayaraj, R.L. Production of Extracellular Pectinase by Bacillus Cereus Isolated from Market Solid Waste. J. Bioanal. Biomed. 2011, 3, 70–75.
- Tripathi, G.D.; Zoya, J.; Singh, A.K. Pectinase production and purification from Bacillus subtilis isolated from soil. Adv. Appl. Sci. Res. 2014, 5, 103–105.
- Chandra, D. Analgesic effect of aqueous and alcoholic extracts of Madhuka Longifolia (Koeing). Indian J. Pharmacol. 2001, 33, 108–111.
- Makinde, A.I. Effects of inorganic fertilizer on the growth and nutrient composition of Moringa (Moringa oleifera). J. Emerg. Trends Eng. Appl. Sci. 2013, 4, 341–343.
- Cabardo, D.E., Jr.; Portugaliza, H.P. Anthelmintic activity of Moringa oleifera seed aqueous and ethanolic extracts against Haemonchus contortus eggs and third stage larvae. Int. J. Vet. Sci. Med. 2017, 5, 30–34.
- Hagiwara, A.; Hidaka, M.; Takeda, S.; Yoshida, H.; Kai, H.; Sugita, C.; Watanabe, W.; Kurokawa, M. Anti-Allergic Action of Aqueous Extract of Moringa oleifera Lam. Leaves in Mice. Eur. J. Med. Plants 2016, 16, 1–10.
- Rastogi, T.; Buhtda, V.; Moon, K.; Aswar, P.B.; Khadabadi, S.S. Comparative studies on anthelmintic activity of Moringa oleifera and Vitex negundo. Asian J. Res. Chem. 2009, 2, 181–182.
