Several active ingredients are capturing the attention of researchers, with the aim to reduce the intensity of symptoms, slowing down the course of the disease, preventing complications and, consequently, improving the quality of life.
2. Long-Term Consequences and After-effects of COVID Infection
Coronavirus disease 2019 (COVID-19) is caused by a novel coronavirus known as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and it was declared a pandemic by the World Health Organization on 11 March 2020 [
11].
Coronaviruses are single-stranded, positive-sense RNA viruses that can infect animals and humans. COVID-19 is transmitted between people through small airborne droplets emanated by an infected individual, personal contact (shaking hands), and by touching infected surfaces [
12]. This disease often causes no symptoms or mild symptoms in the patients affected by the virus; consequently, they usually have a good prognosis. However, many of these cases develop symptoms in a more severe form that can lead to complications that persist long after the infection [
13]. In particular, although COVID-19-associated symptomatology was more evident in individuals with severe disease, individuals with mild and moderate disease also reported a wide range of manifestations after the resolution of the clinical disease [
14].
Since the new SARS-CoV-2 is genetically comparable to previously discovered coronavirus strains, such as SARS-CoV and MERS-CoV, it is highly expected that the consequences in patients recovered from COVID-19 are analogous to those of SARS and MERS [
15]. Thus, a careful evaluation of the data available in follow-up studies of these infections could provide a useful scenario for identifying effective therapeutic protocols in the treatment of long-COVID syndromes.
SARS-CoV-2 infection mostly affects the respiratory system [
8] with complications ranging from mild fatigue to severe forms requiring long-term oxygen therapy or even lung transplantation [
16].
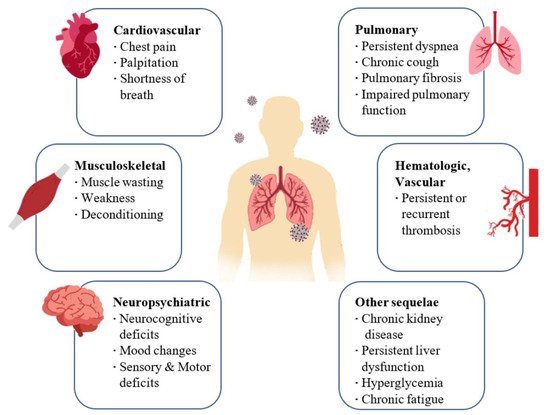
The primary pulmonary manifestations of SARS-CoV-2 include hypoxemia, dyspnea, and cough while severe ones include hypoxemic respiratory failure and ARDS. ARDS may progress into pulmonary fibrosis, which in turn leads to irreversible impairment of respiratory function. Respiratory manifestations typical of post-COVID syndrome include chronic cough and persistent dyspnea [
17].
Some patients develop important neuropsychiatric and musculoskeletal symptoms of COVID-19 including cerebrovascular accidents, olfactory and gustatory impairments, delirium, and myalgia. Some of the neuropsychiatric and musculoskeletal symptoms of post-COVID syndrome include sleep abnormalities, encephalopathy, chronic headache, delirium, brain fog, and small joint arthritis [
18].
Regarding cardiovascular effects, during the acute phase of the infection, patients may report symptoms of shortness of breath, chest pain, and palpitations. These symptoms may persist up to 6 months after infection. Coagulopathies, thrombotic events that may become recurrent or persistent, hyperglycemia, acute kidney injury, and hepatocellular damage have also been observed (
Figure 1) [
17,
19].
Figure 1. Long-term consequences and aftereffects of COVID-19 infections.
These different COVID-19 symptoms reflect the ability of SARS-CoV-2 to infect different types of human cells [
20].
Like other coronaviruses, SARS-CoV-2 shows four structural proteins, known as: S (spike), E (envelope), M (membrane), and N (nucleocapsid) protein. In particular, glycoprotein S is assembled as a homotrimer and is introduced in several copies into the virion membrane, giving it a crown-like appearance. This protein binds the receptor human angiotensin-converting enzyme 2 (ACE2) to infect and enter host cells [
20,
21].
Although the ACE2 receptor is widely expressed in different organs, its expression level in the airways is of primary interest in the case of COVID-19 pathophysiology.
A recent study on ACE2 expression throughout the respiratory tract revealed that it is greatest in the sinus and alveolar type II cells, allowing for easy entry for SARS-CoV-2 [
22].
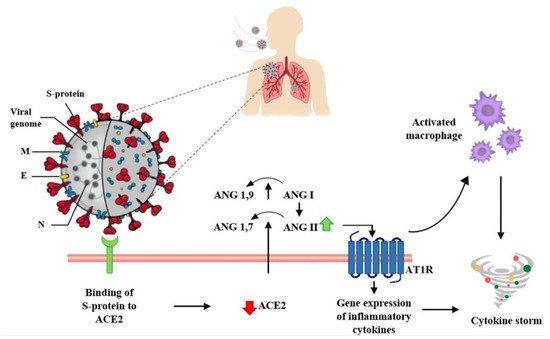
Moreover, the Ang II/AT1R interaction influences the activation of macrophages that contribute to the so-called cytokine storm [
23]. In particular, the ACE2 receptor is a key component of the renin–angiotensin system (RAS). This complex system has a role in the control of blood volume and systemic vascular resistance, which at the same time influences cardiac output and blood pressure [
13]. In detail, angiotensinogen is broken down from renin into inactive angiotensin (Ang I), which is converted into angiotensin II (Ang II) by the angiotensin-converting enzyme (ACE). Ang II binds its own AT1R receptor and controls blood pressure and the immune system, stimulating vasoconstriction and inflammation, as well as tissue injury [
24].
ACE2 counteracts the activity of ACE by converting Ang I into Ang 1–9 (an inert variety of Ang), but it is also able to break down and hydrolyze the vasoconstrictor Ang II into Ang 1–7, which instead exerts a vasodilator effect [
13].
Therefore, the downregulation of ACE2 receptors due to binding with the viral spike protein leads to an increase in angiotensin II, with consequent harmful pro-inflammatory effects. Ang II, in fact, by interacting with its AT1R receptor, stimulates the gene expression of various inflammatory cytokines [
23] (
Figure 2).
Figure 2. Interaction between SARS-CoV-2 and the Renin–Angiotensin System. SARS-CoV-2 enters host cells through the interaction of its spike protein with the ACE2 receptor. The downregulation of ACE2 receptors results in a decrease in the cleavage of angiotensin I and angiotensin II at Ang 1–9 and Ang 1–7, respectively. Ang II, through interaction with the AT1R receptor, stimulates the gene expression of various inflammatory cytokines and also influences the activation of macrophages that contribute to the “cytokine storm”.
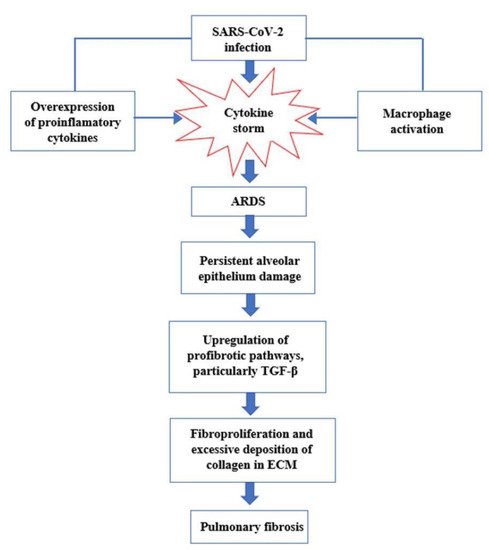
This cytokine storm has been hypothesized to contribute to the development of acute respiratory distress syndrome (ARDS) after COVID-19 infection [
25]. In fact, it has been observed that patients with severe manifestations of COVID-19 often progress to ARDS with permanent scarring of lung tissue and respiratory issue persisting extensively after recovery [
2,
26]. In several autopsy reports, bilateral diffusion of alveolar damage with fibromyxoid cell exudates, pneumocytes desquamation, and hyaline membrane formations have been observed [
27].
The pathological evolution of ARDS consists of three phases: exudative, proliferative, and fibrotic. In the exudative phase, the extra presence of proinflammatory cytokines (IL-1β, TNF, and IL-6) leads to the influx of neutrophils into the lung tissue and the breakdown of the endothelial–epithelial barrier, with consequent loss of fluids in the alveolar spaces and respiratory distress. This phase is followed by the fibroproliferative phase, in which fibrocytes, fibroblasts, and myofibroblasts accumulate in the alveolar compartment, leading to excessive deposition of extracellular components of the matrix (ECM) including fibronectin, collagen I, and collagen III [
28], in order to promote tissue repair.
Although mechanical ventilation (MV) is the most important adjuvant therapy for ARDS, it can worsen lung damage [
29] since besides inducing the secretion of transforming growth factor β1, it also activates collagen synthesis and inhibits collagenase production [
30]. A further problem following mechanical ventilation is respiratory muscle dysfunction: respiratory muscle weakness is approximately two times limb muscle weakness after 1 day of mechanical ventilation, and sepsis, muscle immobilization, and steroids contribute to weakness acquired in intensive care units (ICU) [
31].
A fraction of survivors from ARDS progress to pulmonary fibrosis, which is characterized by the inability of the lungs to rebuild the damaged alveolar epithelium, persistence of fibroblasts, and disproportionate deposition of collagen and other extracellular components of the matrix [
32]. Normally, once the normal lung architecture is rebuilt, the temporary ECM is removed and the fluid from pulmonary edema in the alveolar areas is eliminated as well. However, if ARDS is not managed quickly enough, persistent lung damage will drive uncontrolled fibroproliferation through upregulation of the profibrotic pathways and downregulation of the antifibrotic pathways: among the various profibrotic pathways, transforming growth factor-beta (TGF-β) is the most important mediator and its expression is effectively upregulated in the lungs following SARS-CoV-2 infection [
22,
33].
The activation of TGF-β leads to the deposition of extracellular matrix proteins, stimulation of fibroblast chemotactic migration, and fibroblast to myofibroblast transition (
Figure 3) [
10].
Figure 3. Key events in the progression of cytokine storm to acute respiratory distress syndrome (ARDS) and pulmonary fibrosis.
It is also suggested that following the dysregulation in immunological mechanisms developed as a consequence of COVID-19, an immunosuppressive state occurs to avoid progression to organ damage, especially after the acute hyperinflammatory phase. A prolonged stage of immunosuppression, however, can increase the risk of secondary bacterial and fungal infections [
28,
34].
A significant proportion of survivors from COVID-19 infection showed impaired lung function 6 months after recovery. This is important, not only for the long-term follow-up of these patients, but also to underline the persistent respiratory failure that can result from SARS-CoV-2 infection. Studies of previous coronavirus infections indicate that patients may develop a permanent impairment that lasts for months or even years after infection [
6,
35,
36]. Among the results of the pulmonary function tests, the decrease in the diffusion capacity of carbon monoxide was more evident [
37]. Weakness of the respiratory muscles, development of fibrosis, thrombosis, and angiopathies, particularly those associated with previous diseases and follow-up processes in intensive care units, are just some of the risk factors leading to a worsening of lung function [
34,
36].
3. Management of Patients with Post-COVID Syndrome and after Effects of SARS-CoV-2 Infection
Considering the events occurring after the infection, several classes of active ingredients may be useful in relieving the effects of infection in the airways (Table 1)
|
Drug
|
Category
|
Mode of Action
|
References
|
|
Flavonoids (luteolin, apigenin, kaempferol, fisetin, quercetin, genistein and epigallocatechin gallate)
|
Mast cell level
Stabilizers
|
Anti-inflammatory and mast cell stabilizing effects
|
[40, 42, 43]
|
|
Antihistamine drugs (olopatadine, rupatadine and ketotifen)
|
Mast cell level
stabilizers
|
Anti-inflammatory and mast cell stabilizing effects
|
[40, 42, 44]
|
|
Clarithromycin
|
Mast cell level
Stabilizers
|
Anti-inflammatory and mast cell stabilizing effects
|
[41, 45]
|
|
Dexamethasone
|
Corticosteroids
|
Anti-inflammatory action
|
[48]
|
|
Ciclesonide
|
Corticosteroids
|
Anti-inflammatory action
|
[53]
|
|
Azithromycin
|
Antibiotics
|
Inhibit the proliferation of fibroblasts, reduce the production of collagen and the levels of TGF-β
|
[57]
|
|
Pirfenidone
|
Antifibrotic
|
Inhibit the synthesis of collagen induced by TGF-β; suppresses the production of TNF-α, IFN-γ, IL-1β and IL-6; suppresses the differentiation of fibroblasts associated with TGF-β
|
[61]
|
|
Curcumin
|
Antifibrotic
|
Decreasing the expression of the TGF-β II receptor (TGF-ß RII), as well as in directly reducing the expression of the TGF-β protein and its mRNA
|
[22]
|
|
N-Acetylcysteine (NAC)
|
Antioxidants
|
Inhibits virus replication and expression of pro-inflammatory molecules. boosting a type of cell in your immune system that attacks infections
|
[63]
|
|
GSH
|
Antioxidants
|
Blocks viral replication
through redox state
modulation
|
[64]
|
|
Molnupiravir
|
Antivirals
|
Inhibits the replication of SARS-CoV-2,
acting on the enzyme that the virus
uses to generate copies of itself by introducing
errors into its genetic code
|
[66]
|
|
Zofin
|
Derived from human amniotic fluid
|
Suppressor of
cytokine activation
|
[67]
|
|
Ampion
|
Biological Drug
|
Modulate inflammatory
cytokine levels
|
[69]
|
Recently, Ampio Pharmaceuticals launched a phase I randomized study to evaluate the safety, tolerability, and efficacy of nebulized Ampion in improving the clinical outcomes of 40 patients hospitalized with COVID-19 infections, with persistent respiratory symptoms. Details on the study will be published as soon as they are ready [69] Ampion is the low molecular weight filtrate of human serum albumin and as an immunomodulatory agent with anti-inflammatory effects; it has the potential to modulate inflammatory cytokine levels related to COVID-19 disease and respiratory complications, such as respiratory distress syndrome. acute (ARDS). Administration of Ampion to patients by inhalation allows the drug to reach the target site directly and attenuate lung inflammation [12].
6. Conclusions
Post-COVID syndrome is a new condition that can adversely affect quality of life, regardless of age and the presence of pre-existing diseases. Unfortunately, it is not yet possible to know which patients are most at risk of developing long-term consequences and whether these problems will solve, improve, or become permanent. This review examined the reports of the scientific community on the long-term consequences of COVID-19 and its after-effects, particularly in the lung, as the main site of infection, and possible treatment options useful for alleviating its symptoms. Active ingredients demonstrating a biological logic in the treatment of post-COVID sequelae have been reported, concluding that the most appropriate type of formulation for their administration is inhalation, allowing for
the release of the drug directly on the site of action with a reduction in dose and systemic side effects. Considering also that pulmonary fibrosis has been reported as one of the most serious consequences, the development of new in vitro experimental models, able to faithfully recreate the infection, will help scientists and pharmaceutical companies around the world to develop therapeutic strategies for similar conditions; although, further studies are needed to overcome the limitations of these techniques.