Recently, Otto et al. attributed a role to the type-2-DM-related hyperglycemic inflammatory micromilieu in the acquisition of malignancy-associated alterations in premalignant pancreatic ductal epithelial cells, thus providing new insights into how hyperglycemia might promote PC initiation [
16]. It is well-known that EMT of pancreatic ductal epithelial cells develops in correlation with hyperglycemia or macrophages [
17,
18]. Moreover, hyperglycemia aggravates microenvironment hypoxia, accelerates EMT, and then promotes the metastatic ability of PC. PC is generally hypoxic due to its avascular morphology, and PC cells express high levels of HIF-1α and MMP-9 for promoting tumor growth, invasion and metastasis in a hypoxic environment [
19]. In addition, the accumulation of HIF-1α induced by hyperglycemia might promote pancreatic glycolysis to facilitate cancer progression [
20]. Zhou et al. reported that the high-glucose microenvironment accelerated PC growth [
21]. With regard to the VAs, Guo et al. reported that isoflurane promoted glucose metabolism through upregulation of
miR-21 and suppressed mitochondrial oxidative phosphorylation in ovarian cancer cells [
22]. Dong et al. reported that dezocine, an opioid analgesic, promoted glucose metabolism and impaired the proliferation of lung cancer cells [
23]. However, Codd et al. reported that opioid agonists did not elevate blood glucose and lacked an insulin-reducing effect [
24]. Han et al. reported that indometacin, an inhibitor of cyclooxygenase (COX)-2, ameliorated high-glucose-induced proliferation and invasion via upregulation of E-cadherin in PC cells [
25]. Current laboratory data on the effect of anesthesia on glucose metabolism in PC are limited, and further investigation is required.
Insulin resistance, hyperinsulinemia, hyperglycemia, and chronic inflammation are the mechanisms of type-2-DM-associated PC [
15]. Recently, type 2 DM was shown to reduce the likelihood of cancer survival, and was significantly correlated with comorbidity and poor prognosis in patients undergoing PC surgery [
15]. In addition, metformin may lower the probability of PC. By contrast, insulin therapy may amplify the probability of PC [
15]. In another study, approximately 85% of PC patients exhibited impaired glucose tolerance associated with DM and had a reduced overall survival rate [
26]. Elderly patients with new-onset DM are at higher risk of developing PC than the general population [
26]. Therefore, new-onset DM and hyperglycemia serve as important screening tools to diagnose asymptomatic PC and improve PC survival [
26]. Sandini et al. reported that preoperative blood glucose ≥ 140 mg/dL was associated with poor long-term outcomes in patients undergoing resection for PC [
27]. Conti et al. reported that anti-diabetic drugs represented a significant protective factor against mortality among older adults with metastatic PC [
28]. However, in a recent meta-analysis study, blood glucose, fasting blood glucose, and glycated hemoglobin (HbA1c) levels were not associated with the survival of patients with PC [
29].
3. Tumor Factors: EMT, Hypoxia-Inducible Factor-1α (HIF-1α), Matrix Metalloproteinases (MMP)-9 Expression, Inflammation, Apoptosis, Autophagy, and Oxidative Stress
3.1. EMT
The development of EMT originates in the conversion of epithelial cells to motile mesenchymal stem cells [
40], which is based on many essential processes involving embryonic progression, tissue formation/fibrosis, and wound repairing [
40]. Moreover, the initiation of EMT contributes to tumor growth, therapy resistance, and tumor spreading [
40]. In the case of high EMT expression in tumors, deterioration of overall outcomes and metastases is inevitable. [
40,
41,
42,
43]. However, research on the direct effects of specific anesthetics on EMT of PC is currently lacking, and further investigation is required.
3.1.1. Laboratory Studies
Anesthesia and analgesia may affect EMT [
25,
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61]. Studies have reported that propofol suppressed EMT in esophageal cancer, choriocarcinoma, breast cancer, thyroid cancer, lung cancer, gastric cancer, hepatocellular carcinoma (HCC), renal cell carcinoma (RCC), prostate cancer, and oral squamous cell carcinoma cells [
45,
46,
47,
48,
49,
50,
51,
52,
53,
54]. By contrast, Ren et al. reported that desflurane induced EMT and metastasis in colorectal cancer through deregulation of the miR-34a/LOXL3 axis [
44]. Opioids promoted EMT in breast and lung cancers via mu- or delta-opioid receptors [
55,
56]. Zhang et al. showed morphine-induced EMT in esophageal carcinoma cells [
57]. However, sufentanil inhibited EMT by acting on NF-κB and Snail signaling pathways to inhibit proliferation and metastasis of esophageal cancer [
58]. Lidocaine suppressed EMT in ovarian cancer cells [
59]. However, high concentrations of levobupivacaine significantly increased EMT in the A549 lung cancer cell line, and enhanced metastasis in mice [
60]. COX-2 inhibitors may suppress EMT in oral squamous cell carcinoma [
61]. Han et al. reported that indometacin reduced the expression levels of MMP-2, MMP-9, and vascular endothelial growth factor (VEGF) by upregulation of E-cadherin, inhibiting proliferation and invasion of PC [
25]. Zheng et al. observed the benefit of EMT inhibition due to the use of chemotherapy in PC treatment [
62]. To the best of our knowledge, NSAIDs may inhibit EMT expression in PC. Propofol may inhibit EMT, but VAs may promote EMT in different cancers. On the other hand, opioids and LAs may induce uncertain effects (both positive and negative) on EMT. Laboratory research on the direct effects of specific anesthetics on EMT in PC is currently lacking. Further investigation is needed (see existing studies in
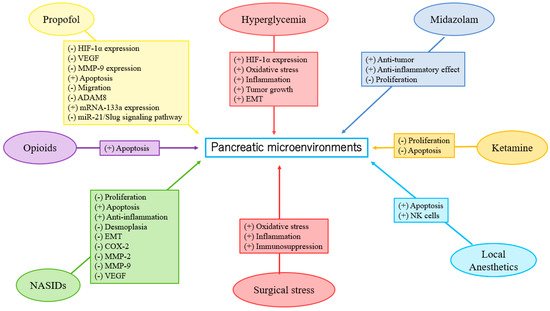
Table 1 and
Figure 1).
Table 1. The existing studies on the effects of anesthetics/analgesics on clinical outcomes and pancreatic microenvironments.
| Type of Anesthetics/Analgesics |
Effects |
Clinical studies
Propofol/VAs |
Propofol was associated with no or low-grade complication compared with desflurane in PC surgery [10]; propofol anesthesia was associated with better survival than desflurane anesthesia in PC surgery [11]. |
| NSAIDs |
In a systematic review of observational studies, there was no signification association between aspirin use and mortality risk in PC [63]; aspirin use reduced risk of PC [64]; aspirin was associated with improved overall survival and improved disease-free survival in PC surgery [65]. |
| Opioids |
High opioid consumption was related to decreased survival rates in newly diagnosed stage IV PC patients [66]; opioid prescription was associated with poor overall survival among PC patients [67]; there was an insignificant relationship between intraoperative opioid use and decreased survival in PC surgery [68]; administration of opioids was associated with prolonged survival in older adult patients with PC [69]. |
| LAs |
Intraoperative administration of intravenous lidocaine was associated with improvement of overall survival in PC patients [12]; intraoperatively epidural ropivacaine infusion was associated with survival improvement in PC patients [70]; perioperative lidocaine administration might be beneficial to the function of NK cells in PC surgery [71]; peridural anesthesia with ropivacaine might improve the oncological outcome of PC patients [72]. |
Experimental studies
Propofol |
Propofol attenuated malignant potential by inhibiting HIF-1α and VEGF expression [73]; PC cell growth was inhibited by propofol via suppression of MMP-9 expression [74]; propofol inhibited migration and induced apoptosis [75]; propofol induced apoptosis in PC cells in vitro [76]; propofol inhibited PC progression by downregulating ADAM8 [77,78,79]; propofol suppressed proliferation and invasion of PC cells by upregulating microRNA-133a expression [80]; propofol inhibited growth and invasion of PC cells through regulation of the miR-21/Slug signaling pathway [81]. |
| NSAIDs |
Indometacin ameliorated high glucose-induced proliferation and invasion by upregulating E-cadherin (EMT) in PC cells [25]; aspirin counteracted PC stem cell features and desmoplasia and gemcitabine resistance [82]; COX-2 inhibition promoted an immune-stimulatory microenvironment in preclinical models of PC [83]; sodium salicylate inhibited proliferation and induced G1 cell cycle arrest in human PC cell lines [84]; indometacin inhibited proliferation and activation of pancreatic stellate cells through the downregulation of COX-2 [85]. |
| Opioids |
Fentanyl decreased gene expression of PC stem cell markers and increased expression of apoptosis-related genes [86]. |
| LAs |
High concentrations of ropivacaine or bupivacaine revealed antiproliferative potency in PC cells [87]. |
| Midazolam |
Midazolam exhibited antitumor (anti-proliferation) and anti-inflammatory effects in a mouse model of PC [88]. |
| Ketamine |
Ketamine significantly inhibited proliferation in PC cells [89]; ketamine significantly inhibited proliferation and apoptosis in PC cells [90]. |
3.1.2. Clinical Studies
Clinical research on the direct effects of specific anesthetics on the EMT of PC is currently lacking. Further investigation is urgently required (Table 1 and Figure 1).
3.2. HIF-1α
A review article showed that HIF-1α expression enhanced PC cell proliferation through multiple mechanisms by inducing neoplastic features and mediating tumorigenic crosstalk between tumor and stromal cells [
91].
3.2.1. Laboratory Studies
Propofol could attenuate PC cells’ malignant potential by inhibiting HIF-1α and VEGF expression [
73]. VAs enhance angiogenesis through HIF-1α activity in prostate and lung cancers [
92]. Isoflurane upregulated the levels of HIF-1α and exerted a protumorigenic effect on a human RCC cell line [
93]. However, in the neuroglioma cell line, sevoflurane decreased HIF-1α expression via
miR-210, while desflurane downregulated HIF1-α and MMP-9 expressions via
miR-138 and
miR-335, respectively [
94]. Opioids were shown to promote tumor angiogenesis in a breast cancer cell by stimulation of δ-opioid receptors in breast cancer cells, leading to activation of HIF-1α and expression of COX-2 via PI3K/Akt stimulation [
95]. However, Koodie et al. reported that morphine suppressed tumor angiogenesis by inhibiting HIF-1α expression in mouse Lewis lung carcinoma cells [
96]. Okamoto et al. showed that HIF-1α activation conferred resistance to lidocaine-induced cell death in the RCC cell line [
97]. Zhou et al. revealed that inhibition of HIF-1α by meloxicam (a selective COX-2 inhibitor) could suppress angiogenesis and enhance apoptosis of HCC cells [
98]. To the best of our knowledge, propofol may reduce HIF-1α expression in PC. Based on the limited data, further investigation is required and encouraged to determine the effects of VAs, LAs, and NSAIDs on HIF-1α expression in PC (
Table 1 and
Figure 1).
Recently, Yue et al. demonstrated that HIF-1α positively regulated miR-212 expression and resulted in pancreatic ductal adenocarcinoma progression [
99]. Propofol inhibited ovarian cancer cells growth and glycolysis by elevating miR-212-5p expression [
100]. Higher miR-212-5p expression showed a neuroprotective effect in rats with isoflurane-induced cognitive dysfunction by inhibiting neuroinflammation [
101]. He et al. reported that δ-opioid receptor activation modified miR-212 expression in the rat kidney under prolonged hypoxia [
102]. Until now, there have been no laboratory studies on the effects of anesthetics on miR-212 expression and PC progression; further investigation is necessary.
3.2.2. Clinical Studies
In a systemic review and meta-analysis, Raji et al. found that miR-212 could be a novel potential biomarker in cancer diagnosis and prognosis [
103]. High levels of miR-212 indicated poor prognosis in PC, and low levels of miR-212 indicated poor prognosis in other cancers [
103]. Until now, there have been no clinical studies on the effects of anesthetics on HIF-1α or miR-212 expression and PC progression; further investigation is necessary.
3.3. MMP-9
MMPs are part of the zinc-dependent proteolytic metalloenzyme family that may play a role in the early diagnosis and prognosis of PC. The higher expression of particular MMPs may also correlate with metastatic disease and/or poorer prognosis [
104,
105,
106,
107]. MMP-9, well-known as one of the most investigated MMPs, corrupts the extracellular matrix components, resulting in pathophysiologic alterations [
108]. Impairment of MMP-9 expression and regulation affects various dysfunctions, including tumorigenesis, and MMP-9 suppression can be targeted in anticancer therapeutics [
108]. Anesthesia may affect MMP-9 expression [
74,
94,
109,
110,
111,
112,
113,
114,
115].
3.3.1. Laboratory Studies
Yu et al. reported that propofol inhibits PC growth by suppressing MMP-9 expression [
74]. Sevoflurane and desflurane inhibited glioma cell proliferation and migration via downregulation of MMP-9 [
94]. Sevoflurane and desflurane reduced the invasion of colorectal and neuroglioma cancer cells through downregulation of MMP-9 [
94,
110]. Moreover, sevoflurane inhibited the proliferation and invasion of HCC cells through downregulation of MMP-9 [
111]. Zhang et al. showed that fentanyl inhibited tumor growth and cell invasion in colorectal cancer by downregulation of miR-182 and MMP-9 expression [
112]. In addition, the antitumor effects of morphine are associated with a reduction in the level of MMP-9 [
113]. Both lidocaine and ropivacaine inhibited TNFα-induced invasion of lung adenocarcinoma cells in vitro by blocking MMP-9 expression [
114]. Based on the limited data, further investigation is needed to clarify the effects of anesthetics and analgesics on MMP-9 expression in PC (
Table 1 and
Figure 1).
3.3.2. Clinical Studies
Wang et al. reported that MMP-9 in the propofol group was significantly lower than in the sevoflurane group in lung cancer patients who received surgery [
109]. In breast cancer patients, Kashefi et al. reported that novel NSAIDs may reduce MMP-2 and MMP-9 expression, which promotes angiogenesis and metastasis [
115]. In summary, propofol may reduce MMP-9 expression in PC. Based on the limited data, further investigation is required to determine the effects of anesthetics and analgesics on MMP-9 expression in PC (
Table 1 and
Figure 1).
3.4. Inflammation, and the Immune System
Inflammation, apoptosis, and autophagy can provide cellular defense, and impairments of these processes (rendering them deficient or overactivated) lead to pathological effects. Inflammation induces secretion of various cytokines and chemokines, and recruits various immune cells in reaction to oxidative stress or infection sites. Reflexively, enhancement of reactive oxygen species (ROS)-generation via inflammatory immune cells provokes oxidative stress and tissue injury. In addition, chronic inflammation not only produces high numbers of inflammatory mediators but also gives rise to oxidative stress [
116]. Inflammatory processes have emerged as key elements in PC development and progression [
117,
118]. The relationship of chronic inflammation and cancer, as revealed in the pioneering work of Rudolf Virchow, has been observed for more than 150 years, especially in PC progression [
118]. However, even in malignancy without preceding inflammation, cancer-induced inflammation, secretions of inflammatory factors, and immune cell infiltration are main characters in tumor initiation and advanced metastasis [
118]. Anesthesia and analgesia may impact cellular immunity and inflammation during surgery, and thus affect cancer outcomes [
119,
120,
121,
122,
123,
124,
125].
3.4.1. Laboratory Studies
A recent laboratory study has shown that propofol enhances anti-inflammatory reactions and stimulatory effects on immune responses, which may be a potential benefit in the prevention of tumor recurrence. However, clinical evidence of the tumor suppression effects is inconclusive. [
126]. Opioids influence the nervous system indirectly, as well as release biological amines that potentially impair innate immunity by suppressing natural killer (NK) cell cytotoxicity. [
127]. However, a mu-opioid receptor (MOR) partial agonist, buprenorphine, intercepted the inhibition of NK cell cytotoxicity and progression caused by surgery in a rat mammary adenocarcinoma cell line [
128]. Additionally, neoplasm is related to inflammation, and anti-inflammatory properties are identified in LAs. LAs may be able to reduce metastasis risk, but the molecular mechanism is not fully understood. [
129]. With regard to NSAIDs, aspirin was associated with a decreased expression of markers for progression, inflammation, and desmoplasia in PC cell lines [
82]. NSAIDs also reduced inflammation and induced apoptosis in rat osteosarcoma cells in vitro [
130]. Thus, NSAIDs may attenuate inflammation in PC. Further laboratory research is necessary (see extant research in
Table 1 and
Figure 1).
3.4.2. Clinical Studies
The surgical treatment of PC is complicated by the prolonged nature of the surgery, the magnitude of the surgical stress, inflammatory response, immunosuppression, anesthesia-/epidural-induced hypotension, and blood loss, all of which cause oxidative stress [
92,
131]. In a retrospective study based on clinical pathological analysis, Huang et al. showed that the survival probability was reduced in patients with TNM stage III to IV, lymph node metastasis, higher CD4
+IL-17
+ level, and lower CD8
+ expression, which implied that the tumor immune microenvironment may affect the outcome of PC [
132]. Recently, Li et al. reported that high systemic immune-inflammation index levels were regarded as negative with regard to PC overall survival and cancer-specific survival [
133]. Yamaguchi et al. demonstrated that propofol reduced the number of CD8
+ T cells, whereas sevoflurane augmented the percentage of regulatory T cells in lung-cancer surgery patients [
121]. Sevoflurane was revealed to devastate multiple pulmonary functions by releasing a series of inflammatory secretions in lung cancer patients undergoing perioperative one-lung ventilation [
134]. However, another clinical study reported that sevoflurane inhibited pulmonary inflammatory cytokines [
135]. Propofol combined with epidural anesthesia and epidural analgesia demonstrated less interference with the immune system (compared to propofol with intravenous analgesia) and led to fast recovery in patients undergoing radical resection of pulmonary carcinoma [
125]. However, in a clinical study, Fant et al. reported that thoracic epidural analgesia with bupivacaine inhibits the neurohormonal but not the acute inflammatory stress response after radical retropubic prostatectomy [
136]. Based on the published data, further investigation is required to determine the effects of anesthetics and analgesics on inflammation and cellular immunity in PC progression (
Table 1 and
Figure 1).