Chile is in the extreme southwestern part of America, and it has an extreme length, of approximately 4300 km that increases to 8000 km considering the Chilean Antarctic Territory. Despite the large extent of its coastal territory and the diversity of geographic environments and climates associated with Chilean coasts, the research on marine resources in Chile has been rather scarce.
1. Introduction
Marine natural products have served as a rich source of new bioactive agents
[1][2][3]. The diversity of marine habitats and unique sea environmental conditions have enabled marine organisms to develop mostly untapped sources of potential drugs with superior chemical novelty
[4]. During the last decades, much effort has been dedicated to isolating and identifying new compounds from marine organisms, with the interesting outcome that many of these derivatives exhibited biological activities
[5][6][7][8][9][10]. Algae are one of the simplest organisms containing chlorophyll, and, therefore, are found in almost every place where there is light to perform photosynthesis, namely in seas, lakes, rivers, animals and plants (as symbiotic species)
[11]. They can be found as colonies of single-celled or multicellular organisms, and in some cases collaborating as simple tissues. Consequently, their size varies from 3–10 µm (unicellular algae) to 70 m long, i.e., giant kelp species growing up to 50 cm per day. Algae are classified into two major groups: microalgae, found both in benthic environments (littoral) and in the ocean (phytoplankton), and macroalgae (marine algae) red, brown and green algae, established in the littoral zone
[12]. Phytoplankton comprise organisms such as diatoms (Bacillariophyta), dinoflagellates (Dinophyta), green and yellow-brown flagellates (Chlorophyta; Prasinophyta; Prymnesiophyta, Cryptophyta, and Rhaphidiophyta) and blue-green algae (Cyanophyta). These photosynthetic organisms play an important role in the productivity of oceans and are at the base of the food chain
[11].
Marine macroalgae have been used in a number of important areas including the food industry
[13], agriculture
[14] and as raw materials on third-generation bioplastics
[15]. However, despite their extended use for decades in traditional medicinal therapies, and the huge number of bioactive compounds that have been extracted and identified, algae are still underrepresented in the pharmaceutical industry. For example, compounds isolated from seaweeds showed interesting biological activities such as: antiprotozoal
[16], antimicrobial
[17][18][19], antifouling
[20][21], anticancer
[22], antileishmanial
[23] and anti-inflammatory properties
[24]. Between these compounds are: terpenoids, sterols, phenols, peptides, polysaccharides, acrylic acid, vitamins, proteins, heterocyclic compounds, chlorophyllides, halogenated ketones and alkanes as well as cyclic polysulfides
[25]. From the large variety of metabolites isolated from algae, the most abundant compounds are terpenes, including monoterpenes, sesquiterpenes and diterpenes. These compounds, which are formed by different numbers of isoprene units (2-methylbuta-1,3-diene), have been found in volatile oils of terrestrial plants as well as in seaweed
[26][27]. All of them possess great potential for further development in pharmacological applications
[28].
It is worth noting that the reason why seaweeds produce such a vast spectrum of secondary metabolites is because they live in nonfriendly environments, and, therefore, they are forced to synthesize protective compounds and to develop protective mechanisms [29]. Abiotic stresses to which algae are exposed include rapid fluctuations in light intensity, temperature, osmotic stress and desiccation, which induce the formation of free radicals and oxidizing agents leading to photodynamic damage [30]. The benthic habitat maps of continental Chile show special topographic characteristics that have joined with coastal currents and winds to enhance the spread of algae, and consequently, around 440 species have been identified [31]. Therefore, the study of Chilean algae is a challenging task, and they are probably a unique source of new compounds with interesting biological activities [32]. Thus, researchers present a review of the literature on terpenes, C15-acetogenins, and furanones as secondary metabolites isolated from Chilean marine algae.
2. Secondary Metabolites Isolated from Chilean Algae
2.1. Terpenoids
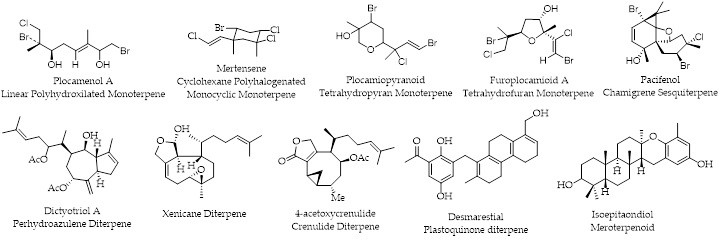
Chilean waters and coasts have turned out to be a fertile source of a variety of marine organisms from which new terpenes have been obtained: Linear Polyhydroxylated Monoterpenes, Cyclohexane Polyhalogenated Monocyclic Monoterpenes, Tetrahydropyran Monoterpenes, Tetrahydrofuran Monoterpenes, Chamigrene Sesquiterpenes, Perhydroazulene Diterpenes, Xenicane Diterpenes, Crenulides Diterpenes, Plastoquinone Diterpenes, and Meroterpenoids.
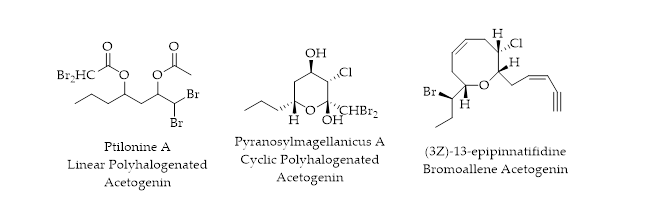
2.2. C15-Acetogenins
Acetogenins are compounds biosynthesized from ethyl acetate or acetyl coenzyme A. Several halogenated C
15-acetogenins, possessing acetylenes, allenes, and oxygen heterocycles, have been isolated from seaweeds
[33]. Both linear and cyclic polyhalogenated C
15-acetogenins have been reported.
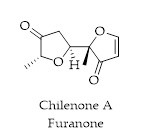
2.3. Furanones
2-Furanone is a heterocyclic organic compound. It is also known as γ-crotonolactone (GCL), as it is formally the lactone derived from γ-hydroxyisocrotonic acid. 4-Hydroxy-2,5-dimethyl-3(2H)-furanone (Furaneol
®, HDMF, also 4-hydroxy-2,5-dimethyl-2,3-dihydrofuran-3-one) was identified for the first time in 1960, as a product of the Maillard reaction or nonenzymatic browning
[34]. Previously, the synthesis and quorum sensing modulating effects of halogenated furanones isolated from the algae and their synthetic analogues have been reported
[35]. Furanones have been obtained from Chilean algae.
This entry is adapted from the peer-reviewed paper 10.3390/md20050337