The gut microbiota composition is important for nutrient metabolism, mucosal barrier function, immunomodulation, and defense against pathogens. Alterations in the gut microbiome can disturb the gut ecosystem. These changes may lead to the loss of beneficial bacteria or an increase in potentially pathogenic bacteria. Furthermore, these have been shown to contribute to the pathophysiology of gastrointestinal and extra-intestinal diseases. Pathologies of the liver, such as non-alcoholic liver disease, alcoholic liver disease, cirrhosis, hepatocellular carcinoma, autoimmune hepatitis, viral hepatitis, and primary sclerosing cholangitis have all been linked to changes in the gut microbiome composition.
1. Introduction
Each individual has a unique gut microbiota profile that regulates many key functions. The gut microbiota is composed of non-pathogenic bacteria, eukaryotic microorganisms, viruses, parasites, and archaea that colonize the gastrointestinal tract [
1].
Bacteroidetes and
Firmicutes constitute 90% of the bacteria in the human digestive tract [
2].
Over the last decade, there has been exponential growth in the literature that has accumulated in describing the gut microbiota and its relationship to both health and disease [
3,
4]. The collective genomes of these bacteria encode more than 150-fold the number of expressive genes than that encoded by the human genome. The gut microbiota encodes over three million genes that produce thousands of beneficial products, whereas the human genome consists of approximately 23,000 genes [
5]. These products, together with host bacteria, are responsible for preserving homeostasis and are key regulators of digestion, metabolism, absorption of nutrients, health, and immunity. A disruption of the symbiotic relationship between the microbiota and the host, or dysbiosis, has been associated with several diseases, including a wide range of liver pathologies. The term dysbiosis can be defined as the disturbance in quantity, variety, and/or location of microorganisms. This can result in the reduction in microbial diversity, which can lead to a disturbance in the balance of the
Firmicutes/
Bacteroidetes ratio, and an increase in symbiotic bacteria that become pathogenic under certain conditions [
6].
There has been a growing number of evidence that demonstrates a bidirectional relationship between the gut microbiota and the liver and many interlinked factors that include: genetics, the environment, and diet, which play a role in contributing to dysbiosis [
7,
8,
9,
10]. The aim of this review was to outline how microbiota and the liver interact with each other. We focused on the general role of the microbiota as well as the role it plays in liver diseases such as nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), cirrhosis, autoimmune hepatitis (AIH), and hepatocellular carcinoma (HCC) as indicated in the current literature. This review also addressed some current regimens that utilize dysbiosis for treating liver pathologies.
2. Role of Gut–Liver Axis in Liver Disease
The term gut–liver axis was created to demonstrate the intimate relationship among the intestine and liver which involves a complex relationship between the gut microbiome, the immune system, and the intestinal barrier [
11]. The liver receives 75% of its blood from the intestines via the portal vein. It also provides feedback to the intestines through the secretion of bile, bile acids, and other mediators.
The interface between the liver and the microbiota is the intestinal epithelium. This structure aids in regulating metabolic functions and selectively permitting the absorption of nutrients while simultaneously acting as a restrictive barrier against any unwanted microbes or microbial products. The selective permeability of the intestinal epithelial barrier is maintained by tight junctions that include E-cadherins, desmosomes, claudins, occludins, and junctional adhesion molecules [
12]. In addition, the intestinal barrier is reinforced by mucins, immunoglobulins, immune cells, and commensal bacteria. Despite the highly specialized epithelium and barriers that modulate the transport across the intestinal mucosa, the disruption of the intestinal barrier can lead to increased intestinal permeability, causing translocation of pathogens, bacteria, and inflammatory cytokines into the portal circulation, which can result in gut inflammation and dysbiosis [
13,
14]. The breakdown of the components of the barrier has been associated with consumption of a high-fat diet, antibiotic use, chronic alcohol abuse, and immune-associated inflammatory disease [
7].
The growing knowledge of the pathophysiology of the gut–liver axis has resulted in a significant number of reviews and evidence that can drive the development of diagnostic, prognostic, and therapeutic tools [
15].
3. Normal Gut Microbiota Composition
The incredibly complex diversity of the gut microbiota comprises many species of microorganisms that include bacteria, bacterial products, yeast, and viruses [
5]. The ability to survey the depth of the gut microbiota has improved due to new high-throughput and sequencing methodologies. There have been 2172 species isolated and thoroughly described taxonomically in human beings [
16]. However, the dominant gut microbial phyla are
Firmicutes,
Bacteroidetes,
Actinobacteria,
Proteobacteria,
Fusobacteria, and
Verrucomicrobia, with the two phyla
Firmicutes and
Bacteroidetes representing 90% of gut microbiota [
5].
The human gut microbiota differs taxonomically and functionally in each part of the gastrointestinal tract. After birth, the human intestine is relatively sterile [
17]. However, increasing evidence suggests that human intestinal microbiota is present before birth [
18]. Maternal microbiota forms the first inoculum after birth; with the initiation of feeding, bacterial colonization is introduced. The microbial diversity increases to form an adult-like microbiota by the end of 3–5 years of life [
18].
The gut microbiota composition is comparatively stable throughout adult life, but it can be altered due to infection, antibiotic use, surgery, age, sex, diet, lifestyle, genetics, environment, and various pathologies [
19]. Each individual has a unique microbiota composition, and thus there is no one healthy composition [
5]. Deschasaux et al., demonstrated that individuals who share the same ethnicity were grouped together, which suggests that they share a similar gut microbiota [
19]. It is also well-known that patients with compromised immune systems or those with liver or inflammatory bowel diseases (IBDs) have an altered microbiota when compared to healthy individuals [
20,
21]. As such, it is crucial to have a better grasp of the gut microbiota in normal physiology and pathophysiology as it provides an enhanced understanding of the microbial alterations in individual patients, which can lead to selectively targeted novel interventions.
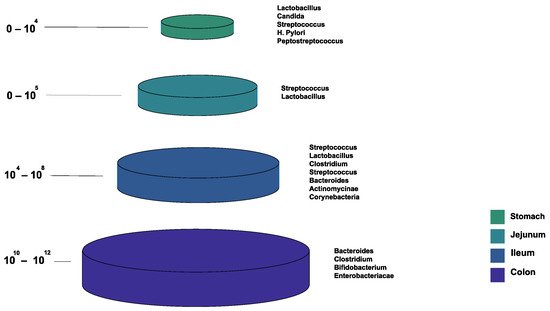
Figure 1 shows the bacterial microbiota composition in various parts of the gut. The gut microbiota is different based on the intestine anatomical regions that vary in terms of physiology, oxygen tension, digestive flow rates (fast in the mouth to the stomach, and slower afterward), and pH [
22]. For example, the small intestine has short transit times (3–5 h), while the large intestine is characterized by slower flow rates and neutral pH, accommodating its large microbial community. The total microbiota load in the intestine is about 10
13–10
14 microorganisms. We can see a quantitative increase in the gradient as we go down the gut, with a predominance of anaerobic bacteria [
17,
22].
Figure 1. The normal composition of the gut microbiota at different locations of the gastrointestinal tract.
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms10051045