The composite microcapsules of alginate/tea tree essential oil have an obvious antibacterial effect on microorganisms in hot spring water, while the composite microcapsules of alginate/chitosan have no antibacterial effect in hot spring water. When the concentration of the cross-linking agent is fixed, the longer the cross-linking time is (10 min > 5 min > 1 min), the longer the release equilibrium time of the essential oil in the microcapsules in the hot spring water is. When the cross-linking time is fixed, the higher the concentration of the cross-linking agent (1 M > 0.5 M > 0.1 M) and the longer the release equilibrium time of the essential oil in the microcapsules in the hot spring water is. When the concentration of the cross-linking agent and the cross-linking time are fixed, the higher the metal activity of the cross-linking agent (Ca > Zn) is and the longer the release equilibrium time of the essential oil in the microcapsules in the hot spring water is.
- alginate
- tea tree essential oil
- microcapsule
- Escherichia coli
- Staphylococcus aureus
1. Microcapsule Technology
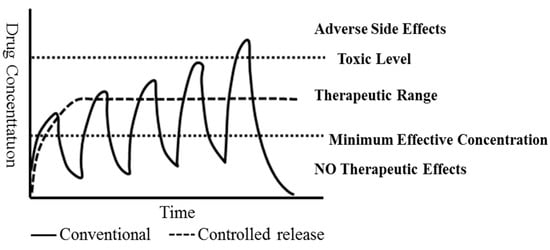
2. Capsule Preparation Technology and Dru-Release System
3. Sodium Alginate, Chitosan, and Tea Tree Essential Oil
4. The Antibacterial Effect of Alginate/Tea Tree Essential Oil Microcapsules and Alginate/Chitosan Microcapsules
5. Discussion on the Release of Microcapsules Obtained with the Same Concentration of Cross-Linking Agent for Different Cross-Linking Time
6. Discussion on the Release with Different Concentrations of Cross-Linking Agents for the Same Cross-Linking Time
This entry is adapted from the peer-reviewed paper 10.3390/w14091391
References
- Stejskal, V.; Vendl, T.; Aulicky, R.; Athanassiou, C. Synthetic and natural insecticides: Gas, liquid, gel and solid formulations for stored-product and food-industry pest control. Insects 2021, 12, 590.
- Labuschagne, P. Impact of wall material physicochemical characteristics on the stability of encapsulated phytochemicals: A review. Food Res. Int. 2018, 107, 227–247.
- Lin, C.L. Study on the Formulation of Capsule Herbicides Alachlor. Master’s Thesis, Chaoyang University of Technology, Taichung, Taiwan, 2006.
- Lin, S.Y.; Kun, B.; Shan, Z.; Shang, W. Microcapsules. Taiwan Sci. 1980, 34, 148–180.
- Wu, P.S. The Preparation and Drug-release Study of Nicotine/Sodium Alginate Composite Microcapsules. Master’s Thesis, Chia Nan University of Pharmacy and Science, Tainan, Taiwan, 2007.
- Alva, G.; Lin, Y.; Liu, L.; Fang, G. Synthesis, characterization and applications of microencapsulated phase change materials in thermal energy storage: A review. Energy Build. 2017, 144, 276–294.
- Zhu, D.Y.; Rong, M.Z.; Zhang, M.Q. Self-healing polymeric materials based on microencapsulated healing agents: From design to preparation. Prog. Polym. Sci. 2015, 49–50, 175–220.
- Simonazzi, A.; Cid, A.G.; Villegas, M.; Romero, A.I.; Palma, S.D.; Bermúdez, J.M. Nanotechnology applications in drug controlled release. Drug Target. Stimuli Sensit. Drug Deliv. Syst. 2018, 81–116.
- Prabakaran, S.; Jeyaraj, M.; Nagaraj, A.; Sadasivuni, K.K.; Rajan, M. Polymethyl methacrylate– graphene oxide drug carrier system for high anti-proliferative cancer drug delivery. Appl. Nanosci. 2019, 9, 1487–1500.
- Guo, X.; Wang, Y.; Qin, Y.; Shen, P.; Peng, Q. Structures, properties and application of alginic acid: A review. Int. J. Biol. Macromol. 2020, 162, 618–628.
- Bilal, M.; Iqbal, H.M.N. Naturally-derived biopolymers: Potential platforms for enzyme immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482.
- Krajewska, B. Application of chitin-and chitosan-based materials for enzyme immobilizations: A review. Enzym. Microb. Technol. 2004, 35, 126–139.
- Jeong, H.J.; Koo, H.N.; Oh, E.Y.; Chae, H.J.; Kim, H.R.; Suh, S.B.; Kim, C.H.; Cho, K.H.; Park, B.R.; Park, S.T.; et al. Nitric oxide production by high molecular weight water-soluble chitosan via nuclear factor-kappaB activation. Int. J. Immunopharmacol. 2000, 22, 923–933.
- Seo, W.G.; Pae, H.O.; Kim, N.Y.; Oh, G.S.; Park, I.S.; Kim, Y.H.; Kim, Y.M.; Lee, Y.H.; Jun, C.D.; Chung, H.T. Synergistic cooperation between watersoluble chitosan oligomers and interferongamma for induction of nitric oxide synthesis and tumoricidal activity in murine peritoneal macrophages. Cancer Lett. 2000, 159, 189–195.
- Lee, P.C.; Syu, F.Y.; Yang, W.J.; Chan, C.F. Antioxidant and antimicrobial activity of high molecular weight water-soluble chitosan. Bull. Hungkuang Instit. Technol. 2012, 67, 1–10.
- Aljazy, N.A.S.; Abdulstar, A.R. Potential effects of natural antioxidants in the treatment of some viral diseases. Al-Qadisiyah J. Agric. Sci. 2021, 11, 1–11.
- Kennedy, D.A.; Lupattelli, A.; Koren, G.; Nordeng, H. Safety classification of herbal medicines used in pregnancy in a multinational study. BMC Complement. Altern. Med. 2016, 16, 1–9.
- Garozzo, A.; Timpanaro, R.; Stivala, A.; Bisignano, G.; Castro, A. Activity of Melaleuca alternifolia (tea tree) oil on Influenza virus A/PR/8: Study on the mechanism of action. Antivir. Res. 2011, 89, 83–88.
- Usachev, E.V.; Pyankov, O.V.; Usacheva, O.V.; Agranovski, I.E. Antiviral activity of tea tree and eucalyptus oil aerosol and vapour. J. Aerosol Sci. 2013, 59, 22–30.
- Gao, Y.Y.; Xu, D.; Wang, R.; Tseng, S.C.G. Treatment of ocular itching associated with ocular demodicosis by 5% tea tree oil ointment. Cornea 2012, 31, 14–17.
- Keragala, R.K.; Kasunsiri, T.D.; Kempitiya, K.S.; Kumarapeli, N.N.; Kumara, K.; Gunathilaka, S.S. A study on the extent, aetiology, and associated factors of dandruff in a group of medical students and the in vitro effects of antidandruff preparations. Sri Lankan J. Infect. Dis. 2020, 10, 134–145.
- Lin, Y.W.; Chang, C.P. Preparation and controlled release properties of alginate microcapsules encapsulated tea-tree oil. J. Hwa Gang Text. 2005, 12, 136–146.
- Cui, H.; Bai, M.; Li, C.; Liu, R.; Lin, L. Fabrication of chitosan nanofibers containing tea tree oil liposomes against Salmonella spp. in chicken. LWT 2018, 96, 671–678.
- Ge, Y.; Tang, J.; Fu, H.; Fu, Y.; Wu, Y. Characteristics, controlled-release, and antimicrobial properties of tea tree oil liposomes-incorporated chitosan-based electrospun nanofiber mats. Fibers Polym. 2019, 20, 698–708.
- Tsai, J.C. Preparation of Active Ingredient-Containing Chitosan/Polycaprolactone Nonwoven Mats Using Electrospinning Technique: In Vitro and In Vivo Evaluations on Wound Healing. Master’s Thesis, National Taiwan University of Science and Technology, Taipei, Taiwan, 2013.
- Su, P.-J.; Xu, X.-L. Antioxidant activity of methanol extracts from Camellia oleifera leaves with different drying treatments. Taiwanese J. Agric. Chem. Food Sci. 2017, 55, 59–65.
- Kikuchi, A.; Kawabuchi, M.; Sugihara, M.; Sakurai, Y. Pulsed dextran release from calcium-alginate gel beads. J. Control. Release 1997, 47, 21–29.
- Jeon, C.; Park, J.Y.; Yoo, Y.J. Novel immobilization of alginic acid for heavy metal removal. Biochem. Eng. J. 2002, 11, 159–166.
