Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Biomedical
Heart rate (HR) is the rate at which the heart pumps and is measured by the number of contractions (beats) of the heart per minute (bpm).
- electrocardiogram
- extramural monitoring
- heart rate
1. Introduction
The heart is an organ that pumps blood through the blood vessels of the circulatory system. This blood carries oxygen and nutrients to the body, while it removes the metabolic waste [1]. Heart rate (HR) is the rate at which the heart pumps and is measured by the number of contractions (beats) of the heart per minute (bpm) [2]. The HR can vary according to the body’s physical needs, such as the need to use oxygen and expel carbon dioxide, and it is modulated by several internal and external factors including genetics, fitness activity, stress or psychological status, diet, bad habits (e.g., smoking or drinking alcohol), medications, hormonal status, environment, and disease/illness [3].
HR is one of the most important vital signs as it can be considered an indicator of the general health status of a subject. For this reason, already since decades, HR is extensively monitored in critical care areas such as the intensive care unit, post-anesthesia care unit, telemetry monitoring units, and emergency departments where patient conditions can rapidly deteriorate [4,5]. Alarms can be generated when the patient’s HR is outside the normal physiological limits [5]. The American Heart Association states that the normal resting HR in adult humans is 60–100 bpm. A cardiac rhythm higher than 100 bpm at rest is defined as tachycardia, while an HR lower than 60 bpm at rest is defined as bradycardia [6].
Another relevant parameter associated with HR is heart rate variability (HRV). HRV is the variation over time of the period between consecutive heartbeats and is predominantly dependent on extrinsic regulation of HR [2]. Several metrics have been investigated to assess HRV. For example, a standard deviation of the cardiac cycle duration lower than 100 ms is considered unhealthy [7] and could involve adverse events such as sepsis and systemic inflammatory response syndrome in low-weight newborns [8] or an acute inflammatory response in COVID-19 patients [9].
In the last decade, the rapid development of sensor, information, and communication technology has provided tremendous opportunities and challenges in the field of extramural monitoring of health status and well-being [10]. Among others, the HR and HRV are one of the most used physiological parameters for extramural monitoring, as in many extramural situations these parameters can be measured by noninvasive and low-cost technologies. For example, monitoring HRV to estimate athletes’ training (mal)adaptation is becoming normal practice to improve athletic performances [11]. HRV is also used to assess the occurrence of mild or severe dehydration levels caused by intense physical activity or by a specific health status (e.g., old age, cardiovascular or kidney diseases, medications, etc.) [12].
Extramural monitoring opens the way to a prolonged and extended evaluation of the heart rhythm, which has several advantages for both the diagnosis of pathology and the prevention of adverse events related to heart disease. For example, reduced HRV is a strong predictor of mortality in patients with infarction and heart failure; therefore, its continuous assessment helps to recognize patients at risk in order to timely intervene with preventive therapy [13]. Moreover, HRV analysis in extramural settings can be used to indirectly infer arousals associated with obstructive sleep apnea syndrome. This novel diagnostic approach enables both mass screening of the population to efficiently identify those who suffer from this disease and long-term monitoring of patients, which are precluded by the traditional diagnostic approach based on the polysomnogram [14]. Finally, continuous and daily HR monitoring helps to improve users well-being and their awareness of their lifestyle. In fact, HR and HRV provide useful information on stress level [15] and sleep quality [16].
The requirements of HR monitoring devices for remote and out-of-hospital (i.e., extramural) applications are different from the devices used in traditional monitoring. In particular, the needs for minimal obtrusiveness, lightweight, and comfort are crucial. Furthermore, the HR estimation provided by the devices must be accurate, especially in devices that are used for medical and diagnostic purposes, even when measurements are made under challenging acquisition conditions (e.g., high-level noise or motion artifacts and loss of contact) and without the supervision of an expert.
2. Sensors Based on Electrical Activity
The pumping function of the heart is the result of a rhythmic contraction and relaxation of approximately 109 muscle cells [17]. This process is controlled by the propagation of biopotentials through the whole cardiac tissue, culminating in a complex electrical pattern. Heart electrical activity begins through spontaneous depolarization of the sinoatrial (SA) node, which is located above the right atrium. This depolarization propagates through the atrial tissue and is transmitted to the ventricles through the atrioventricular node. From the atrioventricular node, the signal enters the bundle of His. This bundle branches into the tree structure of Purkinje fibers, which provides rapid conduction of electrical signals through the ventricles. The initial stimulus of the SA node thus causes depolarization wavefronts, which ultimately activate the entire mass of the ventricles.
2.1. The Electrocardiogram
The heart, from an electrical point of view, can be seen as a dipole. In fact, depolarization and repolarization of cardiomyocytes induce low-intensity electric fields on the surface of the human body. The electrical potential produced by the myocardium is the sum of the potential differences generated by individual cardiomyocytes. These small tensions are recorded through a device called electrocardiograph, which was introduced for the first time in 1903 by Willem Einthoven and Étienne-Jules Marey [18].
The electrical potential generated by the heart’s activity is measured as a potential difference between any pair of electrodes. In non-invasive applications, electrodes are typically made of conductive pads that are attached to the body surface, where the combination of two electrodes forms a lead.
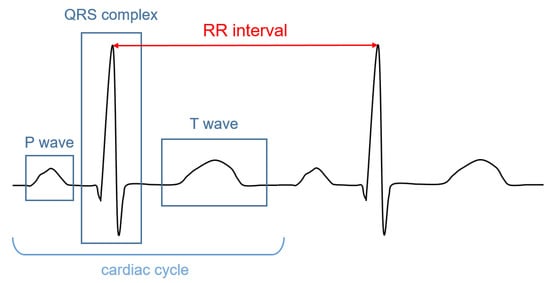
The signal recorded through an electrocardiograph is called electrocardiogram (ECG). It has three main components: the P wave, which corresponds to the depolarization of the atria; the QRS complex; and the T wave, which represent, respectively, the depolarization and the repolarization of the ventricles. The HR (in bpm) can be determined by dividing 60 (seconds per minute) by the RR interval measured in seconds. The RR interval is the distance between two successive R peaks, as shown in Figure 1.

Figure 1. Illustrative ECG signal recorded during one cardiac cycle. The main components are highlighted.
2.2. Instrumentation
The acquisition of the potential differences generated by the electrical activity of the heart in extramural and home monitoring settings is based on portable ECG-based HR monitors. These devices are formed by a handheld sensing unit (e.g., a pad, a watch, a band, a necklace, a t-shirt, wireless sensors, etc.) [19,20,21] sensitive to the electrical field and a unit that captures, processes, and displays or transmits the sensed signals.
The interface between the body and the ECG device is formed by the electrodes. To obtain a stable and high-quality ECG signal, these electrodes have the following requirements: (i) the impedance between the electrode and the skin should be minimized to guarantee a signal with high amplitude; (ii) the electrode should provide stable contact with the skin during the acquisition in order to keep contact during motion; (iii) the electrodes must be biocompatible, avoiding adverse reactions of the skin, and comfortable, even when used for a prolonged time.
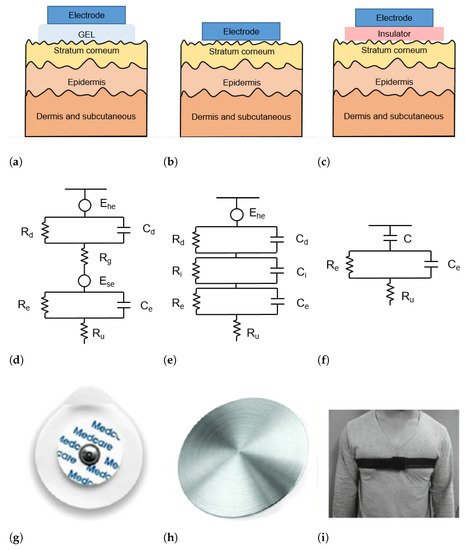
The skin–electrode interface mainly comprises multiple layers of conductive and capacitive coupling, which are complemented by parallel resistor-capacitor (RC) networks connected in series [22]. Depending on which RC networks are predominant, the type and the operating principle of each sensor are defined. In general, the electrodes used for ECG acquisition can be divided into wet, dry, and capacitive electrodes, as depicted in Figure 2.

Figure 2. (a–c) Schematic representation of skin-electrode interface for wet (a), dry (b), and capacitive (c) electrodes; (d–f) equivalent circuit model of skin–electrode interface for wet (d), dry (e), and capacitive (f) electrodes; (g–i) example of wet (g), dry (h), and capacitive (i) electrodes.
2.3. Signal Processing Approaches to Extract the HR
Once the signals have been acquired by the sensing unit, the ECG samples are transmitted to a processing unit (e.g., a smartphone, a smartwatch, a cloud environment, etc.) [44,45], where they can be processed in real time or stored for later processing. Late processing occurs in Holter devices, which are employed especially to diagnose specific cardiac pathology and follow-up cardiopathic patients. In contrast to the delayed processing in Holter devices, fitness trackers, and devices that can generate alarms for emergency assistance typically require real-time analysis of the acquired data.
Most of the methods proposed in the literature to estimate HR from ECG signals are based on the detection of R peaks. Over the years, several methods based on different strategies, such as wavelet transformations, filtering, machine learning, empirical mode decomposition, Markov models, etc., were proposed.
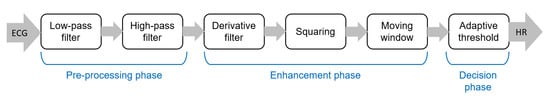
The pipeline of algorithms for R-peak detection can be summarized in three main steps: (i) preprocessing aimed to suppress noise and artifacts; (ii) enhancement of the QRS complexes, reducing at the same time the amplitude of the other waves in the ECG signal in order to make the detection of the R-peak more reliable; and (iii) R-peak detection based on a decision rule approach. The most popular approach for the detection of R peaks is the well-known Pan–Tompkins algorithm [46], which was proposed in 1985 and is still widely employed. The block diagram of this algorithm is reported in Figure 3.

Figure 3. Main steps of the well-known Pan–Tompkins [46] algorithm to detect R-peaks.
Although the Pan–Tompkins algorithm was proposed several decades ago, it is still widely used because it enables efficient detection of QRS complexes, satisfying requirements related to noise rejection, computational load, and accuracy. In fact, the combination of low computational load and high accuracy makes this approach suitable for real-time applications.
ECG signals acquired in extramural and home settings often have a lower quality than signals acquired in a hospital setting. In part, this can be explained by the fact that for long-term extramural monitoring, often dry or capacitive electrodes are used. As mentioned above, these electrodes result in low-amplitude and distorted signals. Furthermore, the acquisition of the ECG during daily life results in signals affected by a large number of motion artifacts [47]. Therefore, there is the need to design ad hoc denoising algorithms aimed at sufficiently increasing the SNR to allow the detection of R-peaks with high accuracy. For example, Peng et al. [48] employed a discrete wavelet transform (DWT) with a moving average to enhance the SNR of ECG signals that were acquired using capacitive electrodes placed over a t-shirt. DWT was also used by Kota et al. [49] to remove noise from signals acquired during physical activity with dry electrodes. Galli et al. [50] presented a denoising algorithm based on a compressive sampling Taylor–Fourier multifrequency (CSTFM) approach, which enables the effective removal of the superimposed noise from ECG signals acquired by low-cost and wearable smartphone-based devices.
Additional sensors (e.g., triaxial accelerometers, multiple biopotential sensors for multiple ECG leads, and electromyography) can also be embedded in portable ECG devices to effectively remove noise. As an example, multiple leads enable the design of a denoising approach based on array processing methods, such as by Lazaro et al., who used Principal Component Analysis (PCA) combined with normalized least mean squares (NLMS) adaptive filtering to effectively reduce the EMG noise from the ECG channels [51].
2.4. Extramural Applications
Handheld ECGs involve the use of dry electrodes (e.g., pads, bands, conductive fabrics integrated into garments) [20,52], or capacitive electrodes embedded in furnishing accessories (e.g., pillows, beds, blankets) [53,54]. To minimize the obtrusiveness of the device, the number of leads used is commonly limited to one. Furthermore, the position of the electrodes is not always standardized; when the electrodes are for instance embedded in objects, the position of the electrodes with respect to the body is quite common [53].
The applications of portable HR monitors based on ECG are many. For instance, wearable ECG bands (e.g., QardioCore (Qardio®, San Francisco, CA, USA)) or adhesive patches (e.g., Zio (iRhythm San Francisco, CA, USA)), are widely employed to track fitness activity [55]. To obtain the most effective fitness workout, the HR should be kept within the optimal boundaries [44]. Monitoring HR and HRV during physical exercise is an important aspect of both sports and rehabilitation medicine because high levels of training or high-performance sports entail a high degree of stress for the human heart that could lead to abnormal or undesired behavior in some athletes. On the other hand, HR monitoring can also be used to quantify the calories burned during exercise [21].
Wearable ECG monitors are also helpful in telemedicine scenarios. For addressing a cardiac rehabilitation condition, Worringham et al. [56] presented a e-Health network consisting of a smartphone, an ECG, and a GPS-based system to remotely monitor the exercise of patients. The system provided a more flexible way to remotely perform unsupervised cardiac rehabilitation, where HR information was transmitted by a programmed smartphone to a server where data could be monitored in real time by qualified medical personnel. In a similar scenario, Baig et al. [57] discuss a system for monitoring the HR of elderly living in remote locations based on wireless textile electrodes. El Attaoui et al. [58] presented a remote ECG monitoring system that can evaluate cardiac electrical activities and detect HR and HRV disorders in patients who suffer from chronic heart disease (e.g., strokes and heart attacks) in order to reduce hospitalizations for examination and follow up. Wearable, wireless, and mobile monitoring systems can provide the best possible solution for telemedicine because they address convenience and comfort; they reduce costs, time, and travel, and enable immediate medical assistance in case of an emergency.
Moreover, by exploiting continuous monitoring, which is made possible by the advent of portable technologies, a higher number of pathologies related to heart rhythm can be diagnosed, especially intermittent diseases such as atrial arrhythmias [59].
2.5. Future Developments
Although ECG-based HR monitoring is widely used in both clinical and extramural settings and several commercial devices are already available on the market, some improvements can still be made. In particular, such improvements include more economic and sustainable manufacturing processes. In fact, the cost associated with the manufacturing of comfortable sensors and ECG devices, as well as the number of waste products (e.g., disposable electrodes), are very high. Therefore, future developments in this field will be oriented to the development of prolonged use and reusability of electrodes in order to reduce both costs and environmental impact.
This entry is adapted from the peer-reviewed paper 10.3390/s22114035
This entry is offline, you can click here to edit this entry!
