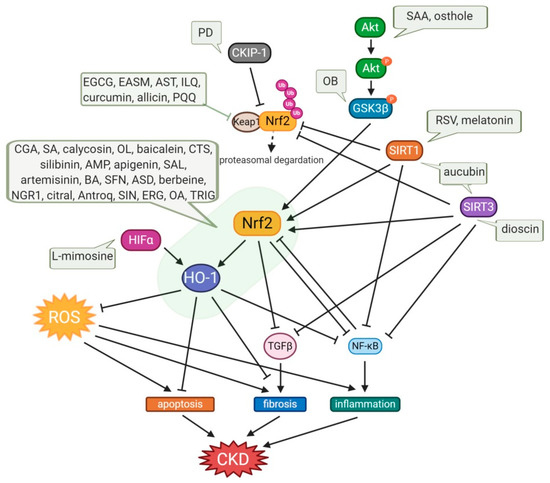
The global burden of chronic kidney disease (CKD) intertwined with cardiovascular disease has become a major health problem. Oxidative stress (OS) plays an important role in the pathophysiology of CKD. The nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant responsive element (ARE) antioxidant system plays a critical role in kidney protection by regulating antioxidants during OS. Heme oxygenase-1 (HO-1), one of the targets of Nrf2-ARE, plays an important role in regulating OS and is protective in a variety of human and animal models of kidney disease. Thus, activation of Nrf2-HO-1 signaling may offer a potential approach to the design of novel therapeutic agents for kidney diseases.
- chronic kidney diseases
- oxidative stress
- Nrf2
- HO-1
- small molecule natural products
1. Introduction
2. Small Molecule Natural Products Activating Nrf2-HO-1 Signaling
| No. | Modulator | Chemical Class and Natural Sources | Experimental Model | Disease Model | Pathobiology Involved | Major Research Outcomes | Molecular Markers | Ref. |
|---|---|---|---|---|---|---|---|---|
| Phenolic compounds | ||||||||
| 1 | Ampelopsin | Flavonoid; Ampelopsis grossedentata | HG-stimulated hGMCs | OS | OS, ECM accumulation | Amelioration of OS and ECM accumulation | ↓ROS, ↓MDA, ↑SOD, ↓Nox2, ↓Nox4, ↓NADPH, ↓FN, ↓Col IV, ↑n-Nrf2, ↑HO-1, |
[36] |
| 2 | Apigenin | Flavonoid; common fruits and vegetables | HG-treated HK-2 cells | Oxidative damage | Oxidative damage | Decrease in apoptosis, inhibition of OS, and inflammatory response | ↓LDH, ↓MDA, ↑SOD, ↑CAT, ↓TNFα, ↓IL-1β, ↓IL-6, ↑Nrf2, ↑HO-1 | [37] |
| 3 | Astaxanthin | Xanthophyll carotenoid; algae, shrimp, lobster, crab, salmon, and other organisms | STZ-injected rat | DKD | ECM accumulation | Amelioration of kidney injury | ↓FN, ↓TGFβ1, ↓ICAM-1 | [38] |
| HG-treated GMCs | Kidney fibrosis | OS | Increase in antioxidative capacity | ↓FN, ↓TGFβ1, ↓ICAM-1, ↑SOD, ↓MDA, ↓ROS, ↓DHE, ↑n-Nrf2, ↓keap1, ↓SOD-1, ↓Nqo1, ↓HO-1 |
||||
| Adriamycin-treated BALB/c mice | FSGS | OS, inflammation | Anti-inflammation, antioxidation | ↓TGFβ1, ↓collagen1, ↓α-SMA, ↓MDA, ↑GSH, ↑SOD, ↑CAT, (serum: ↓IL-1 β, IL-18), ↑Nrf2, ↓NLRP3 | [39] | |||
| 4 | Baicalein | Flavonoid; roots of Scutellaria baicalensis Georgi | Pristine -injected BALB/c mice | LN | OS, inflammation | Attenuation of kidney dysfunction, antioxidation, anti-inflammation, inhibition of MDSC expansion | ↓IL-1b, ↓IL-18, ↓O2¯˙, ↑ GPx, ↑Nrf2, ↑HO-1, ↓ NLRP3, ↓Casp-1, ↓mIL-1 β, ↓p-NF-kB |
[40] |
| LPS-primed spleen-derived MDSCs | OS, inflammation | ↓ROS, ↓IL-1β, ↓IL-18, ↑Nrf2, ↑HO-1, ↓NLRP3, ↓mIL-1β/pro-IL-1β, ↓Casp-1-p20/pro-casp-1-p45, ↓p-NF-kB/NF-kB, ↓Ang-1, ↓p47phox, ↓GP91phox, ↓iNOS |
||||||
| 5 | Calycosin | Isoflavone; root of Astragalus membranaceus | HFD-fed/ STZ-injected SD rat | DKD | Inflammation, OS, fibrosis | Inhibition of inflammatory, oxidative, and fibrotic events | ↓IL-33, ↓ST2, ↓NF-kB p65, ↓TNFα, ↓IL-1 β, ↓IL-6, ↑Nrf2, ↓MDA, ↓TGFβ | [41] |
| 6 | Chlorogenic acid | Cinnamate ester; coffee, fruits, and vegetables | STZ-injected and HFD-fed SD rat | DKD | OS, inflammation | Relieve kidney injury, mitigation of OS, inflammation | ↓MDA, ↑SOD, ↑GSH-Px, ↑n-Nrf2, ↑HO-1, ↓IL-6, ↓TNFα, ↓IL-1 β, ↑c-NF-kB, ↓n-NF-kB, ↑IkBα, ↓p-IkBα, |
[42] |
| HG-treated rat mesangial cell line (HBZY-1) | Mitigation of OS, inflammation, increase in cell proliferation | ↑n-Nrf2, ↑HO-1, ↑c-NF-kB, ↓n-NF-kB, ↑IkBα, ↓p-IkBα, ↓IL-6, ↓TNFα, ↓IL-1 β | ||||||
| 7 | Cryptotanshinone | Quinoid diterpene; Salvia miotiorrhiza bunge | UUO-operated mice | Kidney fibrosis | OS, inflammation | Attenuation of OS and inflammation | ↓collagen-1, ↓FN, ↓CD68, ↓CD3, ↑IkBα, ↓NF-kB p65, ↑SOD2, ↑CAT, ↑GSH, ↓MDA, ↑Nuclear Nrf2, ↓cytosolic Nrf2, ↑HO-1 |
[43] |
| 8 | Curcumin | Curcuminoid; turmeric (Curcuma longa) | 5/6 nephrectomy Wistar rat | CKD | OS, inflammation | Protection of kidney function, antioxidant, anti-inflammation | ↓Nox4, ↑eNOS, ↓nitrotyrosine, ↓MCP-1, ↓Keap-1, ↑Nrf2, ↑GPx-1, ↑CAT, ↑SOD-1, ↓phospho serine D1R |
[44] |
| 0.25% Adenine -diet rat | CKD | OS, inflammation | Amelioration of kidney function and OS | ↓IL-1 β, ↓IL-6, ↓TNFα, ↑cycstatin C, ↓adiponecitn, ↑sclerostin, ↑SOD, ↑Nrf2, ↑GSH reductase. ↓ caspase3 |
[45] | |||
| HG-treated NRK-52E cells | OS | OS | Increase in cell viability, inhibition of EMT | ↑E-cadherin, ↓α-SMA, ↑Nrf2, ↑HO-1 | [46] | |||
| 9 | Epigallocatechin-3 -Gallate | Polyphenol; Dried leaves of tea plant (Camellia sinensis) | STZ-injected mice | DKD | Oxidative damage, inflammation, | Anti-OS | ↓TGFβ1, ↓PAI-1, ↓ICAM-1, ↓VCAM-1, ↓MDA, ↓iNOS, ↓3-NT, ↑Nqo1, ↑HO-1, ↑t-Nrf2, ↑c-Nrf2, ↑n-Nrf2, ↑n-Nrf2/t-Nrf2 | [47] |
| HG-cultured MMC | ↑t-Nrf2, ↑c-Nrf2, ↑n-Nrf2, ↑Nqo1, ↑HO-1, ↓MDA, ↓iNOS, ↓VCAM-1, ↓ICAM-1, ↓COL4, ↓FN |
|||||||
| NZB/W F1 lupus-prone mice | LN | OS | Antioxidant and anti-inflammation | ↑Nrf2, ↓p47phox, ↑Nqo1, ↑HO-1, ↑GPx, ↓CD3, ↓F4/80, ↓NF-kB, ↓NLRP3, ↓IL-1 β, ↓IL-18, ↓casp1-p20, |
[48] | |||
| UUO mice | CKD | OS, inflammation | Kidney function improvement, prevention of OS and inflammation | ↑SOD, ↑CAT, ↑GSH-Px, ↓MPO, ↓TNFα, ↓IL-6, ↓IL-1 β, ↑IkBα, ↓p-IkBα, ↓NF-kB, ↑n-Nrf2, ↑HO-1, ↑t-bilirubin |
[49] | |||
| 10 | Ethyl acetate extract of Saliva miltiorrhiza | Diterpenoids, phenolic compounds, flavonoids, triterpenoids; dried root of Salvia miltiorrhiza Bunge | STZ-injected mice | DKD | Oxidative stress | Antioxidation, attenuation of kidney dysfunction | ↑Nrf2, ↑HO-1, ↑Nqo1, ↓Keap1 | [50] |
| HG-treated SV40-MES-13 MMCs | hyperglycemia | Antioxidation | ↓ROS, ↑Nrf2, ↑HO-1, ↑Nqo1, ↓Keap1 |
|||||
| 11 | Isoliquiritin | Flavonoid glycoside; Chinese licorice (Glycyrrhiza uralensis) | Cationic BSA-injected SD rat | MGN | Inflammation and OS | Antioxidative, anti-inflammatory activities | ↓Keap1, ↑Nrf2, ↓n-Nrf2, ↑c-Nrf2, ↑HO-1, ↑Nqo1, ↓MDA, ↓NO, ↑SOD, ↑CAT, ↑GPx, ↑GSH, ↓NF-kB p65, ↓nuclear NF-kB p65, ↑cyclic NF-kB, ↓IKKb, ↓p-IKKb, ↓TNFα, ↓IL-1 β, ↓COX2, ↓iNOS, ↓p38 MAPK, ↓p-p38 MAPK | [51] |
| 12 | Oleuropein, peracetylatedoleuropein | Secoiridoid; olive leaves, roots, and unprocessed olive drupes | Pristine -injected BALB/c mice | LN | Inflammation and OS | Amelioration of kidney abnormalities, inhibition of proinflammation, antioxidation | ↓MMP-3, ↓iNOS, ↓mPGEs-1, ↓PGE2, ↑Nrf2, ↑HO-1, ↓pSTAT3, ↓NF-kB-p65, ↑IkBα, ↓pp38, ↓pJNK, ↓pERK1/2 ↓NLRP3, ↓ASC, ↓IL-18, ↓ IL-1β, ↓cleaved caspase-1, ↓cleaved caspase 11 |
[52] |
| 13 | Osthole | Coumarin; Fructus Cnidii | 2% adenine suspension -received rat | CKD | Inflammation | Protection of kidney function, antiinflammation | ↓TNFα, ↓IL-6, ↓IL-8, ↓NF-kB/p65, ↓TGFβ1, ↓MCP-1, ↑p-Akt/Akt, ↑Nrf2 |
[53] |
| 14 | Polydatin | Stilbenoid glucoside; Polygonum cuspidatum Sieb.et Zucc | STZ-injected diabetic mice | DKD | OS | Improvement of antioxidative effect and kidney dysfunction | ↑CKIP-1, ↑Nrf2, ↑HO-1, ↑SOD1, ↓FN, ↓ICAM-1, ↓MDA, ↑t-SOD |
|
| HG-treated rat GMCs | ↑Nrf2, ↓Keap1, ↑n-Nrf2, ↓n-CKIP-1, ↑ARE binding activity, ↑HO-1, ↑SOD1, ↓DHE, ↓H2O2, ↓FN, ↓ICAM-1 | |||||||
| 15 | Resveratrol | Phytoalexin; red grapes (Vitis vinifera L.), peanuts (Arachis spp.), berries (Vaccinium spp.) | STZ-induced Wistar rat | DKD | OS | Anti-inflammation, Anti-OS | ↓iNOS, ↓NF-kB, ↓Nrf2, ↓NGAL, ↓IL-1β, ↓IL-6, ↓IL-8, ↓TNFα | [54] |
| 4-hydroxy-2-hexenal-treated mouse cortical collecting duct cells (M1) | OS | ↓nuclear p65, ↑cytosol IkB, ↑SIRT1, ↓Nox4, ↓COX2, ↑AQP2, ↓pERK/ERK, ↓pJNK/JNK, ↓pP38/P38, ↓Nrf2, ↑Keap1 |
[55] | |||||
| 16 | Rotenone | Isoflavonone; seeds and stems of jicama vine plant, the roots of Fabaceae, etc. | UUO-operated mice | Kidney fibrosis | Mitochondrial abnormality | Anti-OS, anti-inflammation, anti-fibrosis | ↓TBARS, ↓HO-1, ↓TNFα, ↓IL-1β, ↓ICAM1, ↓collagen I, ↓FN, ↓α-SMA, ↓PAI-1, ↓collagen III, ↓TGFβ, ↑mtDNA, ↑mtNd1 |
[56] |
| 17 | Salidroside | phenylpropanoid glycoside; plant Rhodiola rosea | HG-treated mouse podocytes | Apoptosis | Apoptosis | Improvement of cell viability | ↓Caspase-9, ↓caspase-3, ↑HO-1, ↑p-ILK/ILK, ↑p-Akt/Akt, ↑p-ERK/ERK, ↑p-JNK/JNK, ↓p-p38/p38, ↑Nrf2 | [57] |
| 18 | Salvianolic acid A | Polyphenol derivative; root of Salvia miltiorrhiza | STZ-injected mice | DKD | OS | Anti-OS | ↓VCAM-1, ↑HO-1, ↓α-SMA, ↓NT, ↓DHE, ↑GPx-1 |
[58] |
| HG-treated HK-2 cells | ↑HO-1, ↓α-SMA, ↓p65, ↓ROS | |||||||
| 5/6 nephrectomized SD rats | CKD | OS | OS attenuation, | ↑t-SOD, ↑GPx, ↑CAT, ↓MDA, ↓ROS, ↓Nox4, ↑p-Akt/Akt, ↑p-GSK3β/GSK3β, ↑p-Nrf2/Nrf2, ↑HO-1 | [59] | |||
| H2O2-treated/LPS-treated HK-2 cells | Cell viability improvement, decrease in OS | ↑t-SOD, ↑GPx, ↑CAT, ↓MDA, ↓ROS, ↓Nox4, ↑p-Akt/Akt, ↑p-GSK3β/GSK3β, ↑n-Nrf2, ↑HO-1, ↓p-NF-kB p65/NF-kB p65, ↓ICAM-1, ↓p-NF-kB p65, ↓ICAM-1, ↑n-Nrf2, ↑HO-1 | ||||||
| 19 | Silibinin | Flavonoliganas: milk thistle seeds | Arsenic -induced rat | CKD | Inflammation | Attenuation of OS, inflammation, and apoptosis | ↓TNFα, ↓iNOS, ↓NO, ↓NF-kB, ↓Caspase-3, ↓NADPH oxidase, ↑Nrf2 |
[60] |
| 20 | Sinapnic acid | Hydroxycinnamic acid; wine, vinegar | STZ-injected rat | DKD | OS, inflammation | Amelioration of OS and inflammation | ↑CAT, ↑GPx, ↑SOD, ↓TNFα, ↓IL-6, ↓NO2, ↓MDA, ↓TFGβ, ↑HO-1, ↑Nrf2, ↓NF-kB, ↑IkBα, ↑Bcl2, ↓Caspase3, ↓Bax |
[61] |
| No. | Modulator | Chemical Class and Natural Sources | Experimental Model | Disease Model | Pathobiology Involved | Major Research Outcomes | Molecular Markers | Ref. |
|---|---|---|---|---|---|---|---|---|
| Non-phenolic compounds | ||||||||
| 1 | Akebia Saponin D | triterpenoid saponin; Dipsaci Radix | STZ-injected mice | DKD | OS, inflammation | Amelioration of kidney damage, inflammation, OS, and apoptosis | ↓TNFα, ↓IL-1β, ↓IL-6, ↓MCP-1, ↓ROS, ↓MDA, ↓LDH, ↑SOD, ↑Bcl2, ↓Bax, ↓cleaved caspase3/caspase3, ↓cleaved caspase9/caspase9, ↑n-Nrf2, ↓p-NF-kB/t-NF-kB, ↑HO-1, ↑Nqo1, ↓p-IkBα/t-IkBα |
[62] |
| HG-treated HK-2 cells | ↓TNFα, ↓IL-1β, ↓IL-6, ↓MCP-1, ↓ROS, ↓MDA, ↓LDH, ↑SOD, ↑Bcl2, ↓Bax, ↓cleaved caspase3/caspase3, ↓cleaved caspase9/caspase9, ↑Nrf2, ↓p-NF-kB/t-NF-kB, ↑HO-1, ↑Nqo1, ↓p-IkBα/t-IkBα |
|||||||
| 2 | Allicin | Diallyl thiosulfinate; garlic (Allium sativum L.) | 5/6 nephrectomy Wistar rat | CKD | Fibrosis, OS | Antihypertensive and antioxidant effects | ↑AT1R, ↑AT2R, ↑Nrf2, ↓Keap1, ↑CAT, ↑SOD, ↓HO-1, ↑eNOS |
[63] |
| 3 | Antroquinonol | Enone; mushroom (Antrodia camphorate) | Adriamycin -injected BALB/c mice | FSGS | OS | Decrease in kidney dysfunction, anti-OS, anti-inflammation | ↓desmin, ↓O2●−, (serum, urine ↓ O2●−, ↓NO), ↓DHE, ↓p47phox, ↑Nrf2, ↑GPx, ↓NF-kB p65, ↓MCP-1, ↓IL-6, ↓CD3, ↓F4/80, ↓Col I, ↓Col III, ↓Col IV, ↓TGFβ1 | [64] |
| 4 | Artemisinin | sesquiterpene lactones; Asteraceae Artemisia annua | STZ-injected rat | DKD | OS | Amelioration of kidney dysfunction and OS | ↓MDA, ↑t-SOD, ↑GPx, ↓TGFβ1, ↑t-Nrf2, ↑n-Nrf2, ↑HO-1, ↑Nqo1 | [65] |
| 5 | Aucubin | iridoid glycoside; leaf of Eucommia ulmoides | HFD-fed and STZ-injected mice | DKD | OS, inflammation | Amelioration of kidney dysfunction, anti-inflammation, anti-OS | ↓FN, ↓collagen IV, ↓MDA, ↑SOD, ↑CAT, ↑GSH/T-GSH, ↓TNFα, ↓IL-6, ↓IL-1β, ↓p65, ↓IkBα, ↑Nrf2, ↑HO-1, ↑Nqo1, ↑FOXO3α, ↓p-FOXO3α/FOXO3α, ↑SIRT1, ↑SIRT3, ↓Ac-FOXO3α/FOXO3α |
[66] |
| 6 | Berberine | isoquinoline alkaloid; Coptidis Rhizoma and Cortex Phellodendri | STZ-injected mice | DKD | OS | Anti-fibrosis | ↓α-SMA, ↓collagen-1, ↑Nrf2, ↑NQO1, ↑HO-1 |
[67] |
| HG-treated NRK 52E cells | EMT | ↓E-cadherin, ↓α-SMA, ↑n-Nrf2, ↑Nqo1, ↑HO-1, ↓p-Smad2, ↓p-Smad3 |
||||||
| 7 | Betulinic acid | pentacyclic triterpenoid; from the outer bark of white birch trees (Betula alba) | STZ-injected SD rat | DKD | OS | Anti-OS | ↓IL-1 β, ↓IL-6, ↓MDA, ↑SOD, ↑CAT, ↑p-AMPK/AMPK, ↓p-IkBα/IkBα, ↓p-NF-kB/NF-kB, ↑Nrf2, ↑HO-1 | [68] |
| 8 | Citral | Terpeonids; Litsea cubeba | Adriamycin -injected BALB/c mice | FSGS | OS | Amelioration of kidney dysfunction, anti-OS, anti-inflammation, anti-apoptosis | ↓O2¯˙, (serum, urine ↓O2¯˙, ↓NO), ↓DHE, ↓p47phox, ↑Nrf2, ↑Nqo1, ↑HO-1, ↓desmin, ↓TUNEL, ↓Casp-3p17, ↓Casp-9p37, ↓Bax/Bcl2, ↓pNF-kB p65, ↓MCP-1, ↓ CD3, ↓F4/80 | [69] |
| LPS-treated RAW 264.7 macrophages | OS | ↓NO, ↓NF-kB, ↓IL-6, ↓TNFα, ↓IL-1β, ↓p-ERK1/2(10min), ↓p-JNK1/2(15,30min) | ||||||
| 9 | Dioscin | Steroid saponin; Dioscoreae rhizoma | 10% fructose -fed mice | CKD | Oxidative damage, lipid metabolism, fibrosis | Inhibition of inflammation, lipid metabolism, OS, kidney fibrosis | ↓MDA, ↑SOD, ↑GSH-Px, ↓α-SMA, ↑SIRT3, ↑SOD2, ↓IL-1β, ↓IL6, ↓TNFα, ↓NF-kB, ↓HMGB1, ↓COX2, ↓c-Jun, ↓c-Fos, ↓SREBP-1c, ↓SCD-1, ↓FASn, ↓p-Akt, ↓p-FoxO1A, ↓ACC, ↑CPT1, ↑Nrf2, ↓Keap1, ↑GST, ↓TGFβ1, ↓p-Smad3, ↑Smad7 |
[70] |
| 10 | Ergone (alisol B 23-acetate, pachymic acid B) | steroid; Polyporus umbellatus, surface layer of Poria cocos, Alisma orientale | AngII- treated HK-2 and conditionally immortalized MPC5 cells | CKD | OS, inflammation, impaired Nrf 2 activation | inhibition of the RAS/Wnt/b-catenin signaling cascade | (HK-2) ↓Snail1, ↓MMP-7, ↓Twist, ↓FSP-1, ↓Col I, ↓Col III, ↓α-SMA, ↓vimentin, ↑E-cadherin, ↓NF-kB, ↓MCP-1, ↓COX2, ↑Nrf2, ↑HO-1 (podocyte) ↓Snail1, ↓MMP-7, ↓Twist, ↓FSP-1, ↑podocin, ↑nephrin, ↑podocalyxin, ↑synaptopodin, ↓desmin, ↑WT1, ↓Akt2, ↓NF-kB, ↓MCP-1, ↓COX2, ↑Nrf2, ↑HO-1 |
[71] |
| 11 | L-mimosine | Amino acid; Mimosa pudica | Rats with remnant kidneys after subtotal nephrectomy (5/6 nephrectomy) | CKD | Fibrosis | Improvement of kidney function, inhibition of fibrosis | ↑HIF-1α, ↑HIF-2α, ↑VEGF, ↑HO-1, ↑GLUT-1, ↓α-SMA, ↓collagen III |
[72] |
| 12 | Melatonin | Endogenous indoleamine, coffee, walnut, etc. | Pristine -injected BALB/c mice | LN | OS, inflammation | Attenuation of OS, inflammation | ↑SIRT1, ↑Nrf2, ↓TNFα, ↓NF-kB, ↓iNOS, ↓NLRP3, ↑CD31 |
[73] |
| 13 | Notoginsenoside R1 | Saponin; Panax notoginseng | db/db mice | DKD | OS | Anti-OS, decrease in apoptosis | ↓Collagen I, ↓TGFβ1, ↑Nrf2, ↑HO-1, ↓Bax/Bcl2, ↓Caspase-3, ↓Caspase-9 | [74] |
| AGEs-treated HK-2 cells | Mitochondria injury | ↓LDH, ↓ROS, ↑n-Nrf2, ↑HO-1, ↓Bax/Bcl2, ↓Cspase-3, ↓Caspase-9, ↓TGFβ1, ↓collagen I |
||||||
| 14 | Obacunone | Triterpenoid limonoid; citrus and other plants of the Rutaceae family | HG-treated NRK-52E cells | OS | OS | Inhibition of OS, mitochondrial injury, and apoptosis | ↑SOD, ↑GSH, ↑CAT, ↓ROS, ↓JC-1 monomer/aggregate, ↑p-GSK3β/GSK3β, ↓n-Fyn, ↑n-Nrf2, ↑Nqo1, ↑HO-1, ↑SOD, ↑GSH, ↑CAT, ↓c-CytC/m-CytC, ↓cleaved caspase3 | [75] |
| 15 | Oleanolic acid | Triterpenoid; olive oil, Phytolacca Americana, Syzygium spp, garlic, etc. | Cyclosporine -treated ICR mice | Chronic nephropathy | Inflammation, fibrosis | Antioxidation, anti-inflammation | ↓α-SMA, ↑HO-1, ↑nuclear/total Nrf2, ↑SOD1, ↓MDA, ↓urinary 8-iso-PGF2α, ↓urine 8-oxo-dG, ↓Bax/Bcl2, ↓active caspase-3 | [76] |
| 16 | Pyrroloquinoline quinone | In soil and foods such as kiwifruit and human breast milk | HG-treated HK-2 cells | OS | OS | Decrease in OS, inflammation and cellular senescence | ↓IL-1β, ↓TNFα, ↓NF-kB, ↓p16, ↓p21, ↓ROS, ↑SOD2, ↑CAT, ↓keap1, ↑Nrf2, ↑HO-1, ↑Nqo1, ↑GST, ↑GPx3, |
[77] |
| 17 | Sinomenine | Alkaloid; Sinomenium acutum | UUO-operated ICR mice | CKD | Fibrosis, OS | Anti-fibrosis, antioxidation | ↑E-cadherin, ↓α-SMA, ↓FN, ↑HO-1, ↑Nqo1, ↑Nrf2, ↑SOD, ↑GPx, ↑CAT, ↑SOD2, ↓p-Smad3, ↓β-catenin |
[78] |
| TGFβ-treated/H2O2-treated HEK293 cells, TGFβ-treated RAW264.7 cells | ↑E-cadherin, ↓α-SMA, ↓FN, ↑HO-1, ↑Nqo1, ↑Nrf2, ↑SOD, ↑GPx, ↑CAT, ↑SOD2, ↓p-Smad3, ↓β-catenin |
|||||||
| 18 | Sulforaphane | Isothiocyanate (organosulfur compound); Cruciferous vegetables such as broccoli, brussels sprouts, and cabbages | STZ-injected and meglumine diatrizoate-injected Wistar rats | DKD, CIN | OS | Renoprotective | ↓MDA, ↓8-oxo-dG, ↑Nrf2, ↑HO-1, ↓IL6, ↑Caspase3 |
[79][80] |
| Meglumine diatrizoate-treated NRK-52E cells | Cell viability | ↑Nrf2, ↑HO-1, ↓IL6 | ||||||
| F344 rat kidneys transplanted Lewis rat | CRAD | OS | OS alleviation, kidney functional and morphological improvements | ↓MDA, ↓8-isoprostane, ↓ox-LDL, ↓8-oxo-dG, ↑SOD, ↑CAT, ↑GPx, ↑GR, ↑ γ-GCS, ↑Nrf2, ↑HO-1, ↑Nqo-1 | [80] | |||
| 19 | Trigonelline | Alkaloid; traditional herbs (especially fenugreek), coffee bean, soybean, and other edible food plants | Oxalate-induced MDCK cells | EMT | Fibrosis | Attenuation of EMT, prevention of cell migration and ROS overproduction, | ↓FN, ↓vimentin, ↓α-SMA, ↑ E-cadherin, ↑ZO-1, ↓MMP9, ↓ROS, ↑Nrf2 |
[81] |
This entry is adapted from the peer-reviewed paper 10.3390/antiox10020258
References
- Carrero, J.J.; Hecking, M.; Chesnaye, N.C.; Jager, K.J. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 151–164.
- Carney, E.F. The impact of chronic kidney disease on global health. Nat. Rev. Nephrol. 2020.
- Liu, M.; Li, X.C.; Lu, L.; Cao, Y.; Sun, R.R.; Chen, S.; Zhang, P.Y. Cardiovascular disease and its relationship with chronic kidney disease. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2918–2926.
- Clausen, P.; Jensen, J.S.; Jensen, G.; Borch-Johnsen, K.; Feldt-Rasmussen, B. Elevated urinary albumin excretion is associated with impaired arterial dilatory capacity in clinically healthy subjects. Circulation 2001, 103, 1869–1874.
- Chan, D.T.; Irish, A.B.; Dogra, G.K.; Watts, G.F. Dyslipidaemia and cardiorenal disease: Mechanisms, therapeutic opportunities and clinical trials. Atherosclerosis 2008, 196, 823–834.
- Holtkamp, F.A.; De Zeeuw, D.; Thomas, M.C.; Cooper, M.E.; De Graeff, P.A.; Hillege, H.J.L.; Parving, H.H.; Brenner, B.M.; Shahinfar, S.; Heerspink, H.J.L. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011, 80, 282–287.
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; Von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B.; et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N. Engl. J. Med. 2016, 375, 323–334.
- Gregg, L.P.; Hedayati, S.S. Management of traditional cardiovascular risk factors in CKD: What are the data? Am. J. Kidney Dis. 2018, 72, 728–744.
- Cruz, M.C.; Andrade, C.; Urrutia, M.; Draibe, S.; Nogueira-Martins, L.A.; Sesso, R.C.C. Quality of life in patients with chronic kidney disease. Clinics 2011, 66, 991–995.
- Lee, H.B.; Yu, M.R.; Yang, Y.; Jiang, Z.; Ha, H. Reactive oxygen species-regulated signaling pathways in diabetic nephropathy. J. Am. Soc. Nephrol. 2003, 14, S241–S245.
- Podkowińska, A.; Formanowicz, D. Chronic kidney disease as oxidative stress-and inflammatory-mediated cardiovascular disease. Antioxidants 2020, 9, 752.
- Noh, H.; Ha, H. Reactive oxygen species and oxidative stress. In Contributions to Nephrology; Karger: Basel, Switzerland, 2011; Volume 170, pp. 102–112.
- Ha, H.; Hwang, I.A.; Park, J.H.; Lee, H.B. Role of reactive oxygen species in the pathogenesis of diabetic nephropathy. Diabetes Res. Clin. Pract. 2008, 82, S42–S45.
- Harman, D. The aging process. Proc. Natl. Acad. Sci. USA 1981, 78, 7124.
- Kim, J.; Kil, I.S.; Seok, Y.M.; Yang, E.S.; Kim, D.K.; Lim, D.G.; Park, J.W.; Bonventre, J.V.; Park, K.M. Orchiectomy attenuates post-ischemic oxidative stress and ischemia/reperfusion injury in mice. A role for manganese superoxide dismutase. J. Biol. Chem. 2006, 281, 20349–20356.
- Stępniewska, J.; Gołembiewska, E.; Dołęgowska, B.; Domański, M.; Ciechanowski, K. Oxidative stress and antioxidative enzyme activities in chronic kidney disease and different types of renal replacement therapy. Curr. Protein Pept. Sci. 2015, 16, 243–248.
- Surh, Y.J.; Kundu, J.K.; Na, H.K. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008, 74, 1526–1539.
- Lv, W.; Booz, G.W.; Fan, F.; Wang, Y.; Roman, R.J. Oxidative stress and renal fibrosis: Recent insights for the development of novel therapeutic strategies. Front. Physiol. 2018, 9, 105.
- Chen, D.Q.; Hu, H.H.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Zhao, Y.Y. Natural products for the prevention and treatment of kidney disease. Phytomedicine 2018, 50, 50–60.
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and oxidative stress in chronic kidney disease-potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int. J. Mol. Sci. 2019, 21, 263.
- Nezu, M.; Suzuki, N.; Yamamoto, M. Targeting the KEAP1-NRF2 system to prevent kidney disease progression. Am. J. Nephrol. 2017, 45, 473–483.
- Lu, M.; Wang, P.; Qiao, Y.; Jiang, C.; Ge, Y.; Flickinger, B.; Malhotra, D.K.; Dworkin, L.D.; Liu, Z.; Gong, R. GSK3β-mediated Keap1-independent regulation of Nrf2 antioxidant response: A molecular rheostat of acute kidney injury to chronic kidney disease transition. Redox Biol. 2019, 26, 101275.
- Yamawaki, K.; Kanda, H.; Shimazaki, R. Nrf2 activator for the treatment of kidney diseases. Toxicol. Appl. Pharmacol. 2018, 360, 30–37.
- Choi, B.H.; Kang, K.S.; Kwak, M.K. Effect of redox modulating NRF2 activators on chronic kidney disease. Molecules 2014, 19, 12727–12759.
- Kanda, H.; Yamawaki, K. Bardoxolone methyl: Drug development for diabetic kidney disease. Clin. Exp. Nephrol. 2020, 24, 857–864.
- Abraham, N.G.; Kappas, A. Heme oxygenase and the cardiovascular–renal system. Free Radic. Biol. Med. 2005, 39, 1–25.
- Li, S.; Qiu, B.; Lu, H.; Lai, Y.; Liu, J.; Luo, J.; Zhu, F.; Hu, Z.; Zhou, M.; Tian, J.; et al. Hyperhomocysteinemia accelerates acute kidney injury to chronic kidney disease progression by downregulating Heme Oxygenase-1 expression. Antioxid. Redox Signal. 2019, 30, 1635–1650.
- Demirogullari, B.; Ekingen, G.; Guz, G.; Bukan, N.; Erdem, O.; Ozen, I.O.; Memis, L.; Sert, S. A comparative study of the effects of hemin and bilirubin on bilateral renal ischemia reperfusion injury. Nephron Exp. Nephrol. 2006, 103, e1–e5.
- Chang, T.T.; Chen, Y.A.; Li, S.Y.; Chen, J.W. Nrf-2 mediated heme oxygenase-1 activation contributes to the anti-inflammatory and renal protective effects of Ginkgo biloba extract in diabetic nephropathy. J. Ethnopharmacol. 2020, 266, 113474.
- Di Noia, M.A.; Van Driesche, S.; Palmieri, F.; Yang, L.M.; Quan, S.; Goodman, A.I.; Abraham, N.G. Heme oxygenase-1 enhances renal mitochondrial transport carriers and cytochrome C oxidase activity in experimental diabetes. J. Biol. Chem. 2006, 281, 15687–15693.
- Bolisetty, S.; Traylor, A.; Zarjou, A.; Johnson, M.S.; Benavides, G.A.; Ricart, K.; Boddu, R.; Moore, R.D.; Landar, A.; Barnes, S.; et al. Mitochondria-targeted heme oxygenase-1 decreases oxidative stress in renal epithelial cells. Am. J. Physiol. Ren. Physiol. 2013, 305, F255–F264.
- Kwak, J.Y.; Takeshige, K.; Cheung, B.S.; Minakami, S. Bilirubin inhibits the activation of superoxide-producing NADPH oxidase in a neutrophil cell-free system. Biochim. Biophys. Acta 1991, 1076, 369–373.
- Nath, K.A. Heme oxygenase-1: A provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006, 70, 432–443.
- Lever, J.M.; Boddu, R.; George, J.F.; Agarwal, A. Heme oxygenase-1 in kidney health and disease. Antioxid. Redox Signal. 2016, 25, 165–183.
- Funes, S.C.; Rios, M.; Fernández-Fierro, A.; Covián, C.; Bueno, S.M.; Riedel, C.A.; Mackern-Oberti, J.P.; Kalergis, A.M. Naturally derived Heme-Oxygenase 1 inducers and their therapeutic application to immune-mediated diseases. Front. Immunol. 2020, 11, 1467.
- Dong, C.; Wu, G.; Li, H.; Qiao, Y.; Gao, S. Ampelopsin inhibits high glucose-induced extracellular matrix accumulation and oxidative stress in mesangial cells through activating the Nrf2/HO-1 pathway. Phytother. Res. 2020, 34, 2044–2052.
- Zhang, J.; Zhao, X.; Zhu, H.; Wang, J.; Ma, J.; Gu, M. Apigenin protects against renal tubular epithelial cell injury and oxidative stress by high glucose via regulation of NF-E2-related factor 2 (Nrf2) pathway. Med. Sci. Monit. 2019, 25, 5280–5288.
- Xie, X.; Chen, Q.; Tao, J. Astaxanthin promotes Nrf2/ARE signaling to inhibit hg-induced renal fibrosis in GMCs. Mar. Drugs 2018, 16, 117.
- He, L.; Liu, G.; Shi, Y.; Peng, X.; Liu, H.; Peng, Y. Astaxanthin attenuates adriamycin-induced focal segmental glomerulosclerosis. Pharmacology 2015, 95, 193–200.
- Li, D.; Shi, G.; Wang, J.; Zhang, D.; Pan, Y.; Dou, H.; Hou, Y. Baicalein ameliorates pristane-induced lupus nephritis via activating Nrf2/HO-1 in myeloid-derived suppressor cells. Arthritis Res. Ther. 2019, 21.
- Elsherbiny, N.M.; Said, E.; Atef, H.; Zaitone, S.A. Renoprotective effect of calycosin in high fat diet-fed/STZ injected rats: Effect on IL-33/ST2 signaling, oxidative stress and fibrosis suppression. Chem. Biol. Interact. 2020, 315.
- Bao, L.; Li, J.; Zha, D.; Zhang, L.; Gao, P.; Yao, T.; Wu, X. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-ĸB pathways. Int. Immunopharmacol. 2018, 54, 245–253.
- Wang, W.; Wang, X.; Zhang, X.S.; Liang, C.Z. Cryptotanshinone attenuates oxidative stress and inflammation through the regulation of Nrf-2 and NF-κB in mice with unilateral ureteral obstruction. Basic Clin. Pharmacol. Toxicol. 2018, 123, 714–720.
- Tapia, E.; García-Arroyo, F.; Silverio, O.; Rodríguez-Alcocer, A.N.; Jiménez-Flores, A.B.; Cristobal, M.; Arellano, A.S.; Soto, V.; Osorio-Alonso, H.; Molina-Jijón, E.; et al. Mycophenolate mofetil and curcumin provide comparable therapeutic benefit in experimental chronic kidney disease: Role of Nrf2-Keap1 and renal dopamine pathways. Free Radic. Res. 2016, 50, 781–792.
- Ali, B.H.; Al-Salam, S.; Al Suleimani, Y.; Al Kalbani, J.; Al Bahlani, S.; Ashique, M.; Manoj, P.; Al Dhahli, B.; Al Abri, N.; Naser, H.T.; et al. Curcumin ameliorates kidney function and oxidative stress in experimental chronic kidney disease. Basic Clin. Pharmacol. Toxicol. 2018, 122, 65–73.
- Zhang, X.; Liang, D.; Guo, L.; Liang, W.; Jiang, Y.; Li, H.; Zhao, Y.; Lu, S.; Chi, Z.H. Curcumin protects renal tubular epithelial cells from high glucose-induced epithelial-to-mesenchymal transition through Nrf2-mediated upregulation of heme oxygenase-1. Mol. Med. Rep. 2015, 12, 1347–1355.
- Sun, W.; Liu, X.; Zhang, H.; Song, Y.; Li, T.; Liu, X.; Liu, Y.; Guo, L.; Wang, F.; Yang, T.; et al. Epigallocatechin gallate upregulates NRF2 to prevent diabetic nephropathy via disabling KEAP1. Free Radic. Biol. Med. 2017, 108, 840–857.
- Tsai, P.Y.; Ka, S.M.; Chang, J.M.; Chen, H.C.; Shui, H.A.; Li, C.Y.; Hua, K.F.; Chang, W.L.; Huang, J.J.; Yang, S.S.; et al. Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic. Biol. Med. 2011, 51, 744–754.
- Wang, Y.; Wang, B.; Du, F.; Su, X.; Sun, G.; Zhou, G.; Bian, X.; Liu, N. Epigallocatechin-3-Gallate attenuates oxidative stress and inflammation in obstructive nephropathy via NF-κB and Nrf2/HO-1 signalling pathway regulation. Basic Clin. Pharmacol. Toxicol. 2015, 117, 164–172.
- An, L.; Zhou, M.; Marikar, F.M.M.T.; Hu, X.W.; Miao, Q.Y.; Li, P.; Chen, J. Salvia miltiorrhiza lipophilic fraction attenuates oxidative stress in diabetic nephropathy through activation of nuclear factor erythroid 2-related factor 2. Am. J. Chin. Med. 2017, 45, 1441–1457.
- Liu, Y.; Xu, X.; Xu, R.; Zhang, S. Renoprotective effects of isoliquiritin against cationic bovine serum albumin-induced membranous glomerulonephritis in experimental rat model through its anti-oxidative and anti- inflammatory properties. Drug Des. Dev. Ther. 2019, 13, 3735–3751.
- Castejon, M.L.; Sánchez-Hidalgo, M.; Aparicio-Soto, M.; Montoya, T.; Martín-LaCave, I.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Dietary oleuropein and its new acyl-derivate attenuate murine lupus nephritis through HO-1/Nrf2 activation and suppressing JAK/STAT, NF-κB, MAPK and NLRP3 inflammasome signaling pathways. J. Nutr. Biochem. 2019, 74.
- Huang, T.; Dong, Z. Osthole protects against inflammation in a rat model of chronic kidney failure via suppression of nuclear factor-κB, transforming growth factor-β1 and activation of phosphoinositide 3-kinase/protein kinase B/nuclear factor (erythroid-derived 2)-like 2 signaling. Mol. Med. Rep. 2017, 16, 4915–4921.
- Koca, H.B.; Pektas, M.B.; Koca, S.; Pektas, G.; Sadi, G. Diabetes-induced renal failure is associated with tissue inflammation and neutrophil gelatinase-associated lipocalin: Effects of resveratrol. Arch. Biol. Sci. 2016, 68, 747–752.
- Bae, E.H.; Joo, S.Y.; Ma, S.K.; Lee, J.U.; Kim, S.W. Resveratrol attenuates 4-hydroxy-2-hexenal-induced oxidative stress in mouse cortical collecting duct cells. Korean J. Physiol. Pharmacol. 2016, 20, 229–236.
- Sun, Y.; Zhang, Y.; Zhao, D.; Ding, G.; Huang, S.; Zhang, A.; Jia, Z. Rotenone remarkably attenuates oxidative stress, inflammation, and fibrosis in chronic obstructive uropathy. Mediat. Inflamm. 2014, 2014.
- Lu, H.; Li, Y.; Zhang, T.; Liu, M.; Chi, Y.; Liu, S.; Shi, Y. Salidroside reduces high-glucose-induced podocyte apoptosis and oxidative stress via upregulating heme oxygenase-1 (HO-1) expression. Med. Sci. Monit. 2017, 23, 4067–4076.
- Wu, P.; Yan, Y.; Ma, L.L.; Hou, B.Y.; He, Y.Y.; Zhang, L.; Niu, Z.R.; Song, J.K.; Pang, X.C.; Yang, X.Y.; et al. Effects of the Nrf2 protein modulator salvianolic acid a alone or combined with metformin on diabetes-associated macrovascular and renal injury. J. Biol. Chem. 2016, 291, 22288–22301.
- Wang, J.H.; Zhang, H.F.; Wang, J.H.; Wang, Y.L.; Gao, C.; Gu, Y.T.; Huang, J.; Zhang, Z. Salvianolic acid A protects the kidney against oxidative stress by activating the Akt/GSK-3 β /Nrf2 signaling pathway and inhibiting the NF- B signaling pathway in 5/6 nephrectomized rats. Oxidative Med. Cell. Longev. 2019, 2019.
- Prabu, S.M.; Muthumani, M. Silibinin ameliorates arsenic induced nephrotoxicity by abrogation of oxidative stress, inflammation and apoptosis in rats. Mol. Biol. Rep. 2012, 39, 11201–11216.
- Alaofi, A.L. Sinapic acid ameliorates the progression of streptozotocin (STZ)-induced diabetic nephropathy in rats via NRF2/HO-1 mediated pathways. Front. Pharmacol. 2020, 11.
- Lu, C.; Fan, G.; Wang, D. Akebia Saponin D ameliorated kidney injury and exerted anti-inflammatory and anti-apoptotic effects in diabetic nephropathy by activation of NRF2/HO-1 and inhibition of NF-KB pathway. Int. Immunopharmacol. 2020, 84.
- García Trejo, E.M.Á.; Buendía, A.S.A.; Reyes, O.S.; Arroyo, F.E.G.; García, R.A.; Mendoza, M.L.L.; Tapia, E.; Lozada, L.G.S.; Alonso, H.O. The beneficial effects of Allicin in chronic kidney disease are comparable to Losartan. Int. J. Mol. Sci. 2017, 18, 1980.
- Tsai, P.Y.; Ka, S.M.; Chao, T.K.; Chang, J.M.; Lin, S.H.; Li, C.Y.; Kuo, M.T.; Chen, P.; Chen, A. Antroquinonol reduces oxidative stress by enhancing the Nrf2 signaling pathway and inhibits inflammation and sclerosis in focal segmental glomerulosclerosis mice. Free Radic. Biol. Med. 2011, 50, 1503–1516.
- Zhang, H.; Qi, S.; Song, Y.; Ling, C. Artemisinin attenuates early renal damage on diabetic nephropathy rats through suppressing TGF-β1 regulator and activating the Nrf2 signaling pathway. Life Sci. 2020, 256.
- Ma, B.; Zhu, Z.; Zhang, J.; Ren, C.; Zhang, Q. Aucubin alleviates diabetic nephropathy by inhibiting NF-κB activation and inducing SIRT1/SIRT3-FOXO3a signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. J. Funct. Foods 2020, 64.
- Zhang, X.; He, H.; Liang, D.; Jiang, Y.; Liang, W.; Chi, Z.H.; Ma, J. Protective effects of berberine on renal injury in streptozotocin (STZ)-Induced diabetic mice. Int. J. Mol. Sci. 2016, 17, 1327.
- Xie, R.; Zhang, H.; Wang, X.Z.; Yang, X.Z.; Wu, S.N.; Wang, H.G.; Shen, P.; Ma, T.H. The protective effect of betulinic acid (BA) diabetic nephropathy on streptozotocin (STZ)-induced diabetic rats. Food Funct. 2017, 8, 299–306.
- Yang, S.M.; Hua, K.F.; Lin, Y.C.; Chen, A.; Chang, J.M.; Kuoping Chao, L.; Ho, C.L.; Ka, S.M. Citral is renoprotective for focal segmental glomerulosclerosis by inhibiting oxidative stress and apoptosis and activating Nrf2 pathway in mice. PLoS ONE 2013, 8, e74871.
- Qiao, Y.; Xu, L.; Tao, X.; Yin, L.; Qi, Y.; Xu, Y.; Han, X.; Tang, Z.; Ma, X.; Liu, K.; et al. Protective effects of dioscin against fructose-induced renal damage via adjusting Sirt3-mediated oxidative stress, fibrosis, lipid metabolism and inflammation. Toxicol. Lett. 2018, 284, 37–45.
- Chen, L.; Chen, D.Q.; Wang, M.; Liu, D.; Chen, H.; Dou, F.; Vaziri, N.D.; Zhao, Y.Y. Role of RAS/Wnt/β-catenin axis activation in the pathogenesis of podocyte injury and tubulo-interstitial nephropathy. Chem. Biol. Interact. 2017, 273, 56–72.
- Yu, X.; Fang, Y.; Ding, X.; Liu, H.; Zhu, J.; Zou, J.; Xu, X.; Zhong, Y. Transient hypoxia-inducible factor activation in rat renal ablation and reduced fibrosis with L-mimosine. Nephrology 2012, 17, 58–67.
- Bonomini, F.; Dos Santos, M.; Veronese, F.V.; Rezzani, R. NLRP3 inflammasome modulation by melatonin supplementation in chronic pristane-induced lupus nephritis. Int. J. Mol. Sci. 2019, 20, 3466.
- Zhang, B.; Zhang, X.; Zhang, C.; Shen, Q.; Sun, G.; Sun, X. Notoginsenoside R1 protects db/db mice against diabetic nephropathy via upregulation of Nrf2-mediated HO-1 expression. Molecules 2019, 24, 247.
- Zhou, J.; Wang, T.; Wang, H.; Jiang, Y.; Peng, S. Obacunone attenuates high glucose-induced oxidative damage in NRK-52E cells by inhibiting the activity of GSK-3β. Biochem. Biophys. Res. Commun. 2019, 513, 226–233.
- Hong, Y.A.; Lim, J.H.; Kim, M.Y.; Kim, E.N.; Koh, E.S.; Shin, S.J.; Choi, B.S.; Park, C.W.; Chang, Y.S.; Chung, S. Delayed treatment with oleanolic acid attenuates tubulointerstitial fibrosis in chronic cyclosporine nephropathy through Nrf2/HO-1 signaling. J. Transl. Med. 2014, 12.
- Wang, Z.; Han, N.; Zhao, K.; Li, Y.; Chi, Y.; Wang, B. Protective effects of pyrroloquinoline quinine against oxidative stress-induced cellular senescence and inflammation in human renal tubular epithelial cells via Keap1/Nrf2 signaling pathway. Int. Immunopharmacol. 2019, 72, 445–453.
- Qin, T.; Yin, S.; Yang, J.; Zhang, Q.; Liu, Y.; Huang, F.; Cao, W. Sinomenine attenuates renal fibrosis through Nrf2-mediated inhibition of oxidative stress and TGFβ signaling. Toxicol. Appl. Pharmacol. 2016, 304, 1–8.
- Khaleel, S.A.; Raslan, N.A.; Alzokaky, A.A.; Ewees, M.G.; Ashour, A.A.; Abdel-Hamied, H.E.; Abd-Allah, A.R. Contrast media (meglumine diatrizoate) aggravates renal inflammation, oxidative DNA damage and apoptosis in diabetic rats which is restored by sulforaphane through Nrf2/HO-1 reactivation. Chem. Biol. Interact. 2019, 309.
- Lv, D.; Zhou, Q.; Xia, Y.; You, X.; Zhao, Z.; Li, Y.; Zou, H. The association between oxidative stress alleviation via sulforaphane-induced Nrf2-HO-1/NQO-1 signaling pathway activation and chronic renal allograft dysfunction improvement. Kidney Blood Press. Res. 2018, 43, 191–205.
- Peerapen, P.; Thongboonkerd, V. Protective roles of trigonelline against oxalate-induced epithelial-to-mesenchymal transition in renal tubular epithelial cells: An in vitro study. Food Chem. Toxicol. 2020, 135.
