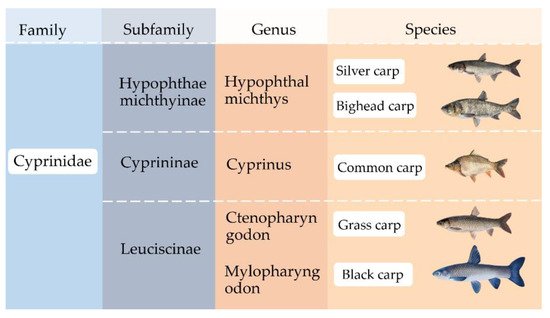
Asian carp is a general designation for grass carp, silver carp, bighead carp, and black carp. These fish species belong to the family Cyprinidae. In 2018, more than 18.5 million tons of Asian carp were produced globally. Asian carp can be used for producing surimi, a stabilized myofibrillar protein concentrate that can be made into a wide variety of products such as imitation crab sticks, fish balls, fish cakes, fish tofu, and fish sausage. Surimi is usually made from marine fish, but Asian carp have been widely used for surimi production in China. The quality of surimi is affected by various factors, including the processing methods and food additives, such as polysaccharides, protein, salt, and cryoprotectant. With an impending shortage of marine fish due to overfishing and depletion of fish stocks, Asian carp have a potential to serve as an alternative raw material for surimi products thanks to their high abundancy, less emissions of greenhouse gases from farming, desirable flesh color, and sufficient gel forming ability.
- Asian carp
- surimi additive
- surimi product
- surimi process
- gelation
1. Introduction
Surimi is a food product widely found in East Asian cuisines, which comes in many shapes and sizes, from fish balls to various kinds of seafood imitation, such as crab sticks. Surimi is made from deboned, minced, and washed fish meat. To be able to imitate the texture of other seafood products, a good gelling property is among the most important characteristics of high-quality surimi. In surimi gelation, myofibrillar proteins, which consist of myosin and actin, play an important role. Although myosin alone can form the gel, actin also cooperates in gelation, which is influenced by the actomyosin ratio. In gelation, heating induces the denaturation of myofibrillar proteins followed by an irreversible aggregation and is cross-linked to form a three-dimensional network [1][2].
Nowadays, there is a wide variety of fish that is currently used as a raw material in surimi production and the majority of them are marine fish. Among those fish species, Alaska pollock is a typical commercial fish used for surimi production as a raw material. It is a cold-water white fish that is available in the North Pacific [3]. The superior gel properties, white flesh, and its availability make it suitable for surimi making. Apart from Alaska pollock, there are several cold-water white fish that are also used in surimi manufacturing, which includes the Arrowtooth flounder, Pacific whiting, and blue whiting [4][5][6][7]. In tropical countries, such as countries in Southeast Asia, tropical fish including threadfin bream, bigeye snapper, and lizardfish are some examples used to produce surimi and surimi products [4]; however, overexploitation of these lean fish species diverts the interest of surimi processing industries towards dark fleshed fish called pelagic fish including sardine, mackerel, etc., but these fish possess weak gelling properties, which are associated with the high lipid, water-soluble protein contents and endogenous proteases firmly attached to the fish muscles [8][9]. Therefore, to overcome the aforementioned problems, freshwater fish could be used as a raw material for surimi production. These freshwater fish have already been used in traditional surimi products, such as fish balls and fish cakes, in China [10].
In general, freshwater fish can be cultured at a low cost and they can attain optimum size in a short time. Therefore, they are gaining more attention to be considered as a potential candidate for surimi manufacturing. Asian carps, namely, bighead carp, grass carp, silver carp, and black carp, are freshwater fish, which can be commonly found in Chinese cuisine. Various recent researches have been performed to improve the gel properties of surimi made from Asian carp [11][12][13][14], and although various collective reports have been available on lean and dark flesh fish surimi gel, no review has been available on the usage of Asian carps in surimi gel preparation and factors affecting gelation. Therefore, this review aims to promote a comprehensive understanding of the Asian carps’ characteristics and their suitability for surimi production. Moreover, factors affecting and solutions to improve the qualities of surimi from Asian carp have also been revisited.
2. Biometric Character of Asian Carp Species
3. Asian Carp Surimi and Surimi Products
3.1. Asian Carp Surimi
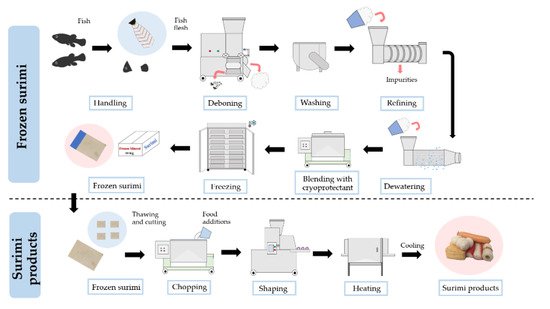
3.2. Surimi Gelation Mechanism
3.3. Traditional Asian Carp Surimi Products
4. The Quality Improvement of Asian Carp Surimi and Surimi Products
4.1. Raw Materials
4.2. Washing Treatments
4.3. Cooking Methods
4.3.1. Traditional Methods
A heating treatment is one of the common processes to induce surimi gel. The quality of surimi products could be dependent on the temperature, heating rate, and heating method. Temperature plays an important role in the denaturation and unfolds of the gel formation. The traditional process of surimi products has been to heat it in a two-step process by a water bath treatment; the first step heated to below 30 °C for the cross-linking of myofibrillar protein, followed by cooking at 90 °C for forming a 3-dimensional protein network and the inactivation of various indigenous enzymes [2][57]. However, water bath heating obtains a heat transfer from the outside surface to the inside of the surimi paste, and causes a slow gel-formation during the fast cooking of surimi products [58][59]. To obtain a fast cooking time with surimi products, several treatments such as microwave heating and radiofrequency have been employed [60][61]. Feng et al. [62] investigated the comparison of microwave heating (100 and 300 w) and water bath heating on the physicochemical properties of fish protein from silver carp. The results showed that the water bath treatment resulted in a surimi gel that had a higher denaturation and aggregation of actomyosin than the microwave; however, the microwave heating (300 w) showed a protective effect on the conformational change of fish protein and improved the quality of surimi. Radiofrequency results in a dielectric heating of the meat product with a low rate of electromagnetic waves. Moreover, radiofrequency has been applied in the surimi heating process to decrease the heating time and reduce nutritional losses, and to improve the quality of surimi products compared with the traditional treatment [61].
4.3.2. Novel Non-Thermal Methods
In addition, non-thermal technology, an acid-induced gel, and 3D printing, have also been developed and applied to the production of Asian carp surimi products [63][64][65]. Non-thermal technology is used as an alternative to traditional heating methods due to its minimal effect on the color, aroma, flavor, and nutrients of food products [66]. The applications of non-thermal technology in surimi products include high-pressure processing, high-intensity ultrasound, and E-beam irradiation.
High-pressure processing, also known as ultra-high-pressure processing or high hydrostatic pressure processing, is a food processing technology that utilizes a high pressure (from 100 MPa up to 900 MPa) [67]. The pressure can cause physicochemical changes and improve the functional properties of food products by enhancing the moisture–protein or protein–protein interactions [68]. The effectiveness of high-pressure processing on the quality of fish products is dependent on the amount of pressure applied and the type of product itself [69]. Liang et al. [63]compared the effectiveness of a high-pressure treatment (100, 200, 300, 400, and 500 MPa) and a two-step heating treatment (40 °C for 30 min and 90 °C for 20 min), on the gel characteristics of surimi from bighead carp. The results showed that the high-pressure treatment gave a higher gel strength and springiness compared with the traditional heating treatment, especially when 500 MPa of pressure was applied.
High-intensity ultrasound is a technique used for improving the physicochemical properties of food products, such as firmness, ripeness, acidity, and sugar content, by applying a low frequency mechanical wave (16–100 kHz) [70]. This technique can also be used for improving the gel properties of surimi products [71]. For example, Gao et al. [12] learned the effects of pre-treatment using high-intensity ultrasound on the gelation properties of surimi from silver carp. It was showed that applying a high-intensity ultrasound pre-treatment before mixing the mince with salt was the most effective pre-treatment mode for promoting the gelation of surimi. The pre-treatment increased the breaking force and prevented deformation of the surimi gel by promoting the formation of non-disulfide bonds and S–S bonds. Moreover, the efficiency of the high-intensity treatment on the gelation properties of surimi is also dependent on the salt contents [72].
Electron beam (E-beam) irradiation is a technique that involves utilizing high-energy electrons for a variety of applications such as pasteurization and sterilization. It is completed by shooting electrons through the product using a linear accelerator [73]. The E-beam generally uses electricity, making it safer compared to gamma ray irradiation using radioisotopes (Co60 or Cs137). The recommended dose for applying E-beam on food products is ≤10 kGy [74]. Electron beam irradiation is, however, not without flaws. Brewer [75] reported a probability that E-beam can cause off-odors and off-flavors in food products. Zhang et al. [76] reported that the application of E-beam irradiation (1–7 kGy) in combination with microwave heating (70 °C) on grass carp surimi produced more volatile compounds than an E-beam irradiation treatment alone, while the control treatment produced the least volatile compounds.
4.4. Functional Ingredients
4.4.1. Polysaccharides
4.4.2. Protein Additives
4.4.3. Microbial Transglutaminase (MTGase)
4.4.4. Salts
4.4.5. Cryoprotectants
4.4.6. Other Food Additives
Oils have been widely used as a texture modifier, color enhancer, or processing aid for surimi production [95]. Numbers have been reported the addition of oils such as peanut oil, soybean oil, and fish oil in silver carp surimi [57][132][133]; however, excessive oil contents could reduce the breaking force due to an interference in the formation of a gel network. Furthermore, the replacement of oil for water may enhance the protein concentration in the matrix of a gel [132][133].
5. Challenges
5.1. Inferior Gel Forming Ability
Asian carp species are known to have a relatively low gel-forming ability. Luo et al. [134] have completed the gel-forming ability of Asian carp species (common carp, grass carp, and silver carp) compared to that of an Alaska pollock, the marine fish species commonly used for surimi production. The result was showed that the Asian carp species had a lower gel-forming ability than an Alaska pollock; however, the Asian carp species still had enough gel-forming ability to be utilized in surimi production [134]. The gel-forming ability of the muscle protein can be affected by several factors such as the muscle sources, protein concentration, heating rate, and heating time. Protein plays an important role in the determining of gel properties and the protein muscle sources can influence the gel-forming ability [2]. Chan et al. [135] reported that the differences in gel-forming abilities of three fish species (cod, herring, and silver hake) were related to the cross-linking abilities of the myosin helical tail. Meanwhile, a number was compared the network structure between marine fish and freshwater fish by using microstructure [136][137]. Riebroy et al. [137] reported that marine fish (Atlantic cod) myosin obtained a higher interconnected and finer network structure than freshwater fish (burbot) myosin. The gel-forming ability was increased due to higher myofibrillar protein concentrations [138]. Asian carp species have white flesh which on average is composed of 16.65% protein content [10], while marine fish such as Alaska pollock flesh is composed of 17.5% proteins [139]. A strong and orderly 3-dimensional surimi gel structure can be achieved when surimi gelation occurs under a slow heating rate and a previous one showed that Asian carp species (common carp, grass carp, and silver carp) required a higher temperature and longer duration for surimi gelation compared with Alaska pollock [134].
5.2. Muddy Odor
Off-odors and off-flavor represent a significant problem for fish and their products, which affects consumer acceptance [140]. The compounds of off-odors are generally derived from enzymatic reactions, microbial activity, lipid oxidation, and environmental or thermal reactions [141]. The off-odors of fish are mainly caused by the odor components of alcohols, aldehydes, and ketones such as hexanal, nonanal, 1-octen-3-ol and 2, 4-heptadienal (E, E) [142]. In addition, the muddy odor or taste in fish is also considered as one of the major problems in carps or other aquaculture fishes. Generally, geosmin and 2-methylisoborneol (MIB) are the main causes of an earthy or muddy odor, which is produced by cyanobacteria and actinomyces [140]. The accumulation of a muddy odor is also dependent on management practices and water quality [143]. On the other hand, a fishy odor is generally related to diatom, chrysophyte, cryptophyte and dinoflagellate. The polyunsaturated fatty acids (PUFAs) present in those algae could result in the production of unpleasant odors [144].
Common methods have been used to remove the off-odor in aquatic products such as adsorption, microcapsules, fermentation, and removal by antioxidants [145],but the removal of off-odor is also dependent on deodorizing agents. Thus, the deodorizing agents should be safe and have a mild odor [146]. The deodorizing of surimi products generally involves washing with saline agents. Currently, several methods have been utilized to remove or reduce the off-odor of Asian carp surimi such as ozone treatments and yeast glucan additions [147][148].
6. Future Directions and Opportunities
Apart from the utilization of Asian carp to substitute for less available marine fish as mentioned earlier, the utilization of Asian carp as a raw material for surimi manufacturing can contribute to the Asian carp problem in the United States as well. Asian carp species, namely, grass carp, silver carp, bighead carp, and black carp are considered as invasive species in the U.S. Their fast reproduction and growth rate are desirable characteristics if they are raised for commercial use but they have no natural predators, making them a threat to native species and the environment. The U.S. government has expended significant effort in attempting to control Asian carp numbers and in preventing their further spread. Using Asian carp as an alternative to marine fish in surimi processing can increase the consumption of Asian carp and lead to a decline in the Asian carp population; however, various things must be undertaken in order to make this strategy work.
First, the customers’ views on Asian carp must be changed. Currently, Asian carp in the U.S. have a bad reputation and people often view Asian carp as a dangerous, inedible and invasive species. Moreover, its off-flavors and intermuscular bones have also made them unappealing. Consumers or farmers should be educated about the nutritive values as well as their cultural practices to control the Asian carp population growth. According to Li et al. [10], more than 60% of American consumers are willing to purchase products from Asian carp after being informed about their benefits. Processing Asian carp into surimi will remove the intermuscular bones and off-flavor, changing the Asian carp into a more appealing and convenient product.
Moreover, there are several other challenges for the production of Asian carp surimi in the US. Li et al. [10] suggested that four reasons have made Asian carp surimi in the U.S. currently unsuccessful. Those reasons are (1) Asian carp is not abundant enough, (2) catching Asian carp has a higher risk, (3) the price of Asian carp is not competitive to other fish, and (4) a lack of skilled workers. The problems with the abundancy and price of Asian carp exist because Asian carp are yet to be raised and produced as a commercial fish. For skilled personnel, experienced personnel from countries familiar with handling Asian carp could be hired from Asian countries, such as China. Asian carp are important freshwater fish species in China and in 2020, the production of Asian carp in China reached 13 million tons. The consumption demand for surimi products in China is increasing, with the production volume of surimi products in China recorded at around 1.3 million tons in 2020 [149]. Asian carp have also been used to produce a variety of surimi products in China, such as fish balls, fish tofu and fish cakes. Due to this increasing demand for surimi products in China, there is pressure increasing for the production of surimi material resources, especially Asian carp. Currently, there is huge progress in the development of aquaculture technology, such as new feeding techniques, breeding methods, and farm management practices [150][151][152], which have led to increases in the production of Asian carp. Asian carp have the potential to be produced in far greater numbers than marine fish.
As the demand for Asian carp in the U.S. grows and Asian carp begins to be raised for consumption, their reputation will be changed from being a dangerous, inedible and invasive species to a nutrient-rich and easy-to-produce commercial fish.
7. Conclusions
Asian carps have a great potential in surimi manufacturing. Their great abundance, appealing white flesh and decent gel-forming ability have made them viable alternatives to marine fish, which are currently used in surimi production. It was summarized the challenges faced in the production of Asian carp surimi along with solutions to improve their quality. The utilization of Asian carp in surimi production also can contribute to solving the threat from Asian carp in the U.S. as well.
This entry is adapted from the peer-reviewed paper 10.3390/foods11091318
References
- Xiong, Y.; Brekke, C. Changes in protein solubility and gelation properties of chicken myofibrils during storage. J. Food Sci. 1989, 54, 1141–1146.
- Sun, X.D.; Holley, R.A. Factors influencing gel formation by myofibrillar proteins in muscle foods. Compr. Rev. Food Sci. Food Saf. 2011, 10, 33–51.
- FAO. The State of World Fisheries and Aquaculture 2020; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; pp. 1–206.
- Guenneugues, P.; Lanelli, J. Surimi resources and market. In Surimi and Surimi Seafood, 3rd ed.; Park, J.W., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 26–53.
- Wasson, D.; Reppond, K.; Babbitt, J.; French, J. Effects of additives of proteolytic and functional properties of arrowtooth flounder surimi. J. Aquat. Food Prod. Technol. 1993, 1, 147–165.
- Reynolds, J.; Park, J.W.; Choi, Y. Physicochemical properties of pacific whiting surimi as affected by various freezing and storage conditions. J. Food Sci. 2002, 67, 2072–2078.
- Trondsen, T. Blue whiting surimi: New perspectives on the market value. Fish. Res. 1998, 34, 1–15.
- Bentis, C.A.; Zotos, A.; Petridis, D. Production of fish-protein products (surimi) from small pelagic fish (Sardinops pilchardusts), underutilized by the industry. J. Food Eng. 2005, 68, 303–308.
- Singh, A.; Benjakul, S. Proteolysis and its control using protease inhibitors in fish and fish products: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 496–509.
- Li, D.; Prinyawiwatkul, W.; Tan, Y.; Luo, Y.; Hong, H. Asian carp: A threat to American lakes, a feast on Chinese tables. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2968–2990.
- Chen, J.; Deng, T.; Wang, C.; Mi, H.; Yi, S.; Li, X.; Li, J. Effect of hydrocolloids on gel properties and protein secondary structure of silver carp surimi. J. Sci. Food Agric. 2020, 100, 2252–2260.
- Gao, X.; Yongsawatdigul, J.; Wu, R.; You, J.; Xiong, S.; Du, H.; Liu, R. Effect of ultrasound pre-treatment modes on gelation properties of silver carp surimi. LWT—Food Sci. Technol. 2021, 150, 111945.
- Liu, C.; Li, W.; Lin, B.; Yi, S.; Ye, B.; Mi, H.; Li, J.; Wang, J.; Li, X. Comprehensive analysis of ozone water rinsing on the water-holding capacity of grass carp surimi gel. LWT—Food Sci. Technol. 2021, 150, 111919.
- Shi, L.; Yin, T.; Huang, Q.; You, J.; Hu, Y.; Jia, D.; Xiong, S. Effects of filleting methods on composition, gelling properties and aroma profile of grass carp surimi. Food Sci. Hum. Wellness 2021, 10, 308–315.
- Martín-Sánchez, A.; Navarro, C.; Pérez-Álvarez, J.; Kuri, V. Alternatives for efficient and sustainable production of surimi: A review. Compr. Rev. Food Sci. Food Saf. 2009, 8, 359–374.
- Panpipat, W.; Chaijan, M.; Benjakul, S. Gel properties of croaker–mackerel surimi blend. Food Chem. 2010, 122, 1122–1128.
- Zhang, L.; Li, Q.; Shi, J.; Zhu, B.; Luo, Y. Changes in chemical interactions and gel properties of heat-induced surimi gels from silver carp (Hypophthalmichthys molitrix) fillets during setting and heating: Effects of different washing solutions. Food Hydrocoll. 2018, 75, 116–124.
- Endoo, N.; Yongsawatdigul, J. Comparative study on chemical and gel-forming properties of surimi from freshwater and marine fish during frozen storage. Food Appl. Biosci. J. 2014, 2, 192–202.
- Núñez-Flores, R.; Cando, D.; Borderías, A.J.; Moreno, H.M. Importance of salt and temperature in myosin polymerization during surimi gelation. Food Chem. 2018, 239, 1226–1234.
- Lanier, T.C. Measurement of surimi composition and functional properties. In Surimi Technology; Lanier, T.C., Ed.; Marcel Dekker: New York, NY, USA, 1992; pp. 123–163.
- Benjakul, S.; Chantarasuwan, C.; Visessanguan, W. Effect of medium temperature setting on gelling characteristics of surimi from some tropical fish. Food Chem. 2003, 82, 567–574.
- Murthy, L.N.; Phadke, G.G.; Jeyakumari, A.; Ravishankar, C. Effect of added calcium and heat setting on gel forming and functional properties of Sardinella fimbriata surimi. J. Food Sci. Technol. 2021, 58, 427–436.
- Lanier, T.C.; Carvajal, P.; Yongsawatdigul, J. Surimi gelation chemistry. In Surimi and Surimi Seafood, 2nd ed.; Park, J.W., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 435–489.
- Miroshnichenko, N.; Balanuk, I.; Nozdrenko, D. Packing of myosin molecules in muscle thick filaments. Cell Biol. Int. 2000, 24, 327–333.
- Niwa, E. Chemistry of surimi gelation. In Surimi Technology; Lanier, T.C., Lee, C.M., Eds.; Marcel Dekker: New York, NY, USA, 1992; pp. 389–428.
- Gilleland, G.; Lanier, T.; Hamann, D. Covalent bonding in pressure-induced fish protein gels. J. Food Sci. 1997, 62, 713–733.
- Benjakul, S.; Visessanguan, W.; Pecharat, S. Suwari gel properties as affected by transglutaminase activator and inhibitors. Food Chem. 2004, 85, 91–99.
- Ooizumi, T. Manufacture of Kamaboko, Chikuwa, Tempura, and Hanpen. In Surimi and Surimi Seafood, 3rd ed.; Park, J.W., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 271–282.
- Lu, H.; Wu, J.; Liu, J. The optimized formulation of silver carp cake. Fish. Sci. 2010, 29, 735–738.
- Xie, S.; Chen, L.; Zhang, Y.; Zheng, B. Effect of ozone on the quality of fish-ball made from silver carp. J. Fujian Agric. For. Univ. 2009, 38, 552–557.
- Xin, M.; Wang, X.; Zhu, Z.; Zeng, Q.; Li, B. Effect of ingredients on gel properties of common carp (Cyprinus carpio) fish ball and changes of fish ball quality during storage. Mod. Food Sci. Technol. 2010, 26, 810–814.
- Dong, Z.; Sun, L.; Qi, X.; Tang, J.; Pan, W.; Yang, R.; Ye, J. Study on processing technology of sea bass/grass carp fish ball. Food Res. Dev. 2019, 40, 103–107.
- Kok, N.; Thawornchinsombut, S.; Park, J.W. Manufacture of fish balls. In Surimi and Surimi Seafood, 3rd ed.; Park, J.W., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 285–299.
- Kok, T.; Park, J.W. Elucidating factors affecting floatation of fish ball. J. Food Sci 2006, 71, E297–E302.
- Wang, R.; Gao, R.; Xiao, F.; Zhou, X.; Wang, H.; Xu, H.; Gong, C.; Huang, P.; Zhao, Y. Effect of chicken breast on the physicochemical properties of unwashed sturgeon surimi gels. LWT—Food Sci. Technol. 2019, 113, 108306.
- Ketnawa, S.; Benjakul, S.; Martínez-Alvarez, O.; Rawdkuen, S. Physical, chemical, and microbiological properties of fish tofucontaining shrimp hydrolysate. Fish. Sci. 2016, 82, 379–389.
- Yamsaengsung, R.; Yaeed, S.; Ophithakorn, T. Vacuum frying of fish tofu and effect on oil quality usage life. J. Food Process Eng. 2017, 40, e12587.
- Ophithakorn, T.; Yaeed, S. Influence of temperature on microstructure and oil content in vacuum frying of fish tofu. Int. J. Adv. Agric. Environ. Eng. 2016, 3, 170–174.
- Lago, A.M.; Vidal, A.C.; Schiassi, M.C.; Reis, T.; Pimenta, C.; Pimenta, M.E. Influence of the addition of minced fish on the preparation of fish sausage: Effects on sensory properties. J. Food Sci. 2017, 82, 492–499.
- Zhang, Q.; Lin, S.; Nie, X. Reduction of biogenic amine accumulation in silver carp sausage by an amine-negative Lactobacillus plantarum. Food Control 2013, 32, 496–500.
- Ghelichi, S.; Shabanpour, B.; Pourashouri, P. Properties of fish sausages containing common carp (Cyprinus carpio) roe oil and defatted roe protein hydrolysate during refrigerated storage. J. Aquat. Food Prod. Technol. 2018, 27, 185–199.
- Ooizumi, T.; Park, J.D. Manufacture of fish sausage. In Surimi and Surimi Seafood, 3rd ed.; Park, J.W., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 301–309.
- Mahmud, A.; Girmatsion, M.; Abraha, B.; Mohammed, J.K.; Yang, F.; Xia, W. Fatty acid and amino acid profiles and digestible indispensable amino acid score of grass carp (Ctenopharyngodon idella) protein concentrate supplemented noodles. J. Food Meas. Charact. 2020, 14, 2370–2379.
- Shikha, F.H.; Hossain, M.I.; Farzana, L. Development of noodles with low cost Silver carp fish (Hypophthalmichthys molitrix) mince. Bangladesh J. Fish. 2020, 32, 107–114.
- Pascual, D.M. Utilization and acceptability of noodles enriched with different levels of fish protein from bighead carp (Aristichthys nobis). Int. Res. J. Basic Appl. Sci. 2016, 25, 389–401.
- Yuan, C.; Fukuda, Y.; Kaneniwa, M.; Chen, S.; Cheng, Y.; Wang, X.; Konno, K. Comparison of gel-forming properties of silver carp (Hypophthalmichthys molitrix) surimi prepared in different seasons. J. Food Sci. 2005, 70, C326–C331.
- He, Y.; Park, J.W.; Yin, T. Biochemical and gelling properties of silver carp surimi as affected by harvesting season and chopping time. J. Food Process. Preserv. 2019, 43, e14247.
- Park, J.W.; Graves, D.; Draves, R.; Yongsawatdigul, J. Manufacture of surimi harvest to frozen block. In Surimi and Surimi Seafood, 3rd ed.; Park, J.W., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 55–95.
- Yongsawatdigul, J.; Wongngam, W.; Khampirat, B. Chemical parameters for traceability of raw material freshness of tropical surimi. J. Aquat. Food Prod. Technol. 2016, 25, 895–904.
- Ocaño-Higuera, V.; Marquez-Ríos, E.; Canizales-Dávila, M.; Castillo-Yáñez, F.; Pacheco-Aguilar, R.; Lugo-Sánchez, M.; García-Orozco, K.; Graciano-Verdugo, A. Postmortem changes in cazon fish muscle stored on ice. Food Chem. 2009, 116, 933–938.
- Gelman, A.; Pasteur, R.; Rave, M. Quality changes and storage life of common carp (Cyprinus carpio) at various storage temperatures. J. Sci. Food Agric. 1990, 52, 231–247.
- Lu, H.; Liu, X.; Zhang, Y.; Wang, H.; Luo, Y. Effects of chilling and partial freezing on rigor mortis changes of bighead carp (Aristichthys nobilis) fillets: Cathepsin activity, protein degradation and microstructure of myofibrils. J. Food Sci. 2015, 80, C2725–C2731.
- Rawdkuen, S.; Sai-Ut, S.; Khamsorn, S.; Chaijan, M.; Benjakul, S. Biochemical and gelling properties of tilapia surimi and protein recovered using an acid-alkaline process. Food Chem. 2009, 112, 112–119.
- Yongsawatdigul, J.; Pivisan, S.; Wongngam, W.; Benjakul, S. Gelation characteristics of mince and washed mince from small-scale mud carp and common carp. J. Aquat. Food Prod. Technol 2013, 22, 460–473.
- Chang, T.; Wang, C.; Yang, H.; Xiong, S.; Liu, Y.; Liu, R. Effects of the acid-and alkali-aided processes on bighead carp (Aristichthys nobilis) muscle proteins. Int. J. Food Prop. 2016, 19, 1863–1873.
- Cheng, F.F.; Yuan, M.L.; Zhao, L.; Su, W.; Wu, R.F. The effect of different washing treatments on grass carp surimi. Adv. Mat. Res. 2013, 19, 1863–1873.
- Jiao, X.; Cao, H.; Fan, D.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Ye, W.; Zhang, H. Effects of fish oil incorporation on the gelling properties of silver carp surimi gel subjected to microwave heating combined with conduction heating treatment. Food Hydrocoll. 2019, 94, 164–173.
- Tadpitchayangkoon, P.; Park, J.W.; Yongsawatdigul, J. Gelation characteristics of tropical surimi under water bath and ohmic heating. LWT—Food Sci. Technol. 2012, 46, 97–103.
- Liang, F.; Zhu, Y.; Ye, T.; Jiang, S.; Lin, L.; Lu, J. Effect of ultrasound assisted treatment and microwave combined with water bath heating on gel properties of surimi-crabmeat mixed gels. LWT—Food Sci. Technol. 2020, 133, 110098.
- Cao, H.; Fan, D.; Jiao, X.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Zhang, H. Heating surimi products using microwave combined with steam methods: Study on energy saving and quality. Innov. Food Sci. Emerg. Technol. 2018, 47, 231–240.
- Wang, L.; Wang, X.; Ma, J.; Yang, K.; Feng, X.; You, X.; Wang, S.; Zhang, Y.; Xiong, G.; Wang, L. Effects of radio frequency heating on water distribution and structural properties of grass carp myofibrillar protein gel. Food Chem. 2021, 343, 128557.
- Feng, D.; Xue, Y.; Li, Z.; Wang, Y.; Xue, C. Effects of microwave radiation and water bath heating on the physicochemical properties of actomyosin from silver carp (Hypophthalmichthys molitrix) during setting. J. Food Process. Preserv. 2017, 41, e13031.
- Liang, Y.; Guo, B.; Zhou, A.; Xiao, S.; Liu, X. Effect of high pressure treatment on gel characteristics and gel formation mechanism of bighead carp (Aristichthys nobilis) surimi gels. J. Food Process. Preserv. 2017, 41, e13155.
- Weng, W.; Zheng, W. Silver carp (Hypophthalmichthys molitrix) surimi acid-induced gel extract characteristics: A comparison with heat-induced gel. Int. J. Food Prop. 2015, 18, 821–832.
- Wang, L.; Zhang, M.; Bhandari, B.; Yang, C. Investigation on fish surimi gel as promising food material for 3D printing. J. Food Eng. 2018, 220, 101–108.
- Zhang, Z.H.; Wang, L.H.; Zeng, X.A.; Han, Z.; Brennan, C.S. Non-thermal technologies and its current and future application in the food industry: A review. Int. J. Food Sci. Technol. 2019, 54, 1–13.
- Xu, Y.; Xu, X. Modification of myofibrillar protein functional properties prepared by various strategies: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 458–500.
- Ma, X.-S.; Yi, S.-M.; Yu, Y.-M.; Li, J.-R.; Chen, J.-R. Changes in gel properties and water properties of Nemipterus virgatus surimi gel induced by high-pressure processing. LWT—Food Sci. Technol. 2015, 61, 377–384.
- Montiel, R.; Cabeza, M.C.; Bravo, D.; Gaya, P.; Cambero, I.; Ordóñez, J.A.; Nuñez, M.; Medina, M. A comparison between E-beam irradiation and high-pressure treatment for cold-smoked salmon sanitation: Shelf-life, colour, texture and sensory characteristics. Food Bioproc. Technol. 2013, 6, 3177–3185.
- Amiri, A.; Sharifian, P.; Soltanizadeh, N. Application of ultrasound treatment for improving the physicochemical, functional and rheological properties of myofibrillar proteins. Int. J. Biol. Macromol. 2018, 111, 139–147.
- Fan, D.; Huang, L.; Li, B.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Zhang, H. Acoustic intensity in ultrasound field and ultrasound-assisted gelling of surimi. LWT—Food Sci. Technol. 2017, 75, 497–504.
- Gao, X.; Xie, Y.; Yin, T.; Hu, Y.; You, J.; Xiong, S.; Liu, R. Effect of high intensity ultrasound on gelation properties of silver carpsurimi with different salt contents. Ultrason. Sonochem. 2021, 70, 105326.
- Jaczynski, J.; Park, J.W. Microbial inactivation and electron penetration in surimi seafood during electron beam processing. J.Food Sci. 2003, 68, 1788–1792.
- Jaczynski, J.; Park, J.W. Physicochemical changes in Alaska pollock surimi and surimi gel as affected by electron beam. J. Food Sci. 2004, 69, FCT53–FCT7.
- Brewer, M. Irradiation effects on meat flavor: A review. Meat Sci. 2009, 81, 1–14.
- Zhang, H.; Wang, W.; Wang, H.; Ye, Q. Effect of e-beam irradiation and microwave heating on the fatty acid composition and volatile compound profile of grass carp surimi. Radiat. Phys. Chem. 2017, 130, 436–441.
- Singh, A.;Benjakul, S.;Prodpran, T.;Nuthong, P. Effect of psyllium (Plantago ovata Forks) husk on characteristics, rheological and textural properties of threadfin bream surimi gel. Foods 2021, 10, 1181.
- Ramírez, J.A.; Uresti, R.M.; Velazquez, G.; Vázquez, M. Food hydrocolloids as additives to improve the mechanical and functional properties of fish products: A review. Food Hydrocoll. 2011, 25, 1842–1852.
- Hunt, A.; Getty, K.; Park, J.W. Roles of starch in surimi seafood: A review. Food Rev. Int. 2009, 25, 299–312.
- Smith, A.M. The biosynthesis of starch granules. Biomacromolecules 2001, 2, 335–341.
- Liu, H.; Nie, Y.; Chen, H. Effect of different starches on colors and textural properties of surimi-starch gels. Int. J. Food Prop. 2014, 17, 1439–1448.
- Wu, M.; Hamann, D.; Lanier, T. Rheological and calorimetric investigations of starch-fish protein systems during thermal processing. J. Texture Stud. 1985, 16, 53–74.
- Yang, H.; Park, J.W. Effects of starch properties and thermal-processing conditions on surimi–starch gels. LWT—Food Sci Technol. 1998, 31, 344–353.
- Wu, Y.; Chang, S.; Tan, Y.; Zhang, Y.; Mahmoud, B.S. Carp muscle protein patterns and textural properties as affected by starch additions to the mince protein gels made from wild grass carp (Ctenopharyngodon Idella), silver carp (Hypophthalmichthys molitrix) and bigmouth buffalo carp (Ictiobus cyprinellus). J. Food Nutr. Sci. 2020, 2, 1–18.
- Wang, Y.; Jin, Y.Z.; Cheng, Y.D. Effect of adding silver carp surimi on gelatinization characteristics of wheat flour dough during microwave heating. Adv. Mat. Res. 2012, 396–398, 1382–1388.
- Campo, L.; Tovar, C. Influence of the starch content in the viscoelastic properties of surimi gels. J. Food Eng. 2008, 84, 140–147.
- Hazarika, B.J.; Sit, N. Effect of dual modification with hydroxypropylation and cross-linking on physicochemical properties of taro starch. Carbohydr. Polym. 2016, 140, 269–278.
- Liu, H.; Ramsden, L.; Corke, H. Physical properties and enzymatic digestibility of hydroxypropylated ae, wx, and normal maize starch. Carbohydr. Polym. 1999, 40, 175–182.
- Salehi, F. Characterization of new biodegradable edible films and coatings based on seeds gum: A review. J. Packag. Technol. Res. 2019, 3, 193–201.
- Mi, H.; Li, Y.; Wang, C.; Yi, S.; Li, X.; Li, J. The interaction of starch-gums and their effect on gel properties and protein conformation of silver carp surimi. Food Hydrocoll. 2021, 112, 106290.
- Benjakul, S.; Visessanguan, W.; Tanaka, M.; Ishizaki, S.; Suthidham, R.; Sungpech, O. Effect of chitin and chitosan on gellingproperties of surimi from barred garfish (Hemiramphus far). J. Sci. Food Agric. 2001, 81, 102–108.
- Alishahi, A.; Aïder, M. Applications of chitosan in the seafood industry and aquaculture: A review. Food Bioproc. Technol. 2012, 5, 817–830.
- Yin, T.; Yao, R.; Ullah, I.; Xiong, S.; Huang, Q.; You, J.; Hu, Y.; Shi, L. Effects of nanosized okara dietary fiber on gelation properties of silver carp surimi. LWT—Food Sci. Technol. 2019, 111, 111–116.
- Jannat-Alipour, H.; Rezaei, M.; Shabanpour, B.; Tabarsa, M.; Rafipour, F. Addition of seaweed powder and sulphatedpolysaccharide on shelf-life extension of functional fish surimi restructured product. J. Food Sci. Technol. 2019, 56, 3777–3789.
- Xue, S.; He, L. Optimization of adding polysaccharides from chicory root based on fuzzy mathematics to improve physicochemical properties of silver carp surimi balls during storage. J. Food Process. Preserv. 2021, 45, e15307.
- Park, J.W.; Ooizumi, T.; Hunt, A.L. Ingredient technology for surimi and surimi seafood. In Surimi and Surimi Seafood, 3rd ed.; Park, J.W., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 453–491.
- Chang, C.; Niu, F.; Su, Y.; Qiu, Y.; Gu, L.; Yang, Y. Characteristics and emulsifying properties of acid and acid-heat induced egg white protein. Food Hydrocoll. 2016, 54, 342–350.
- Zhou, X.; Chen, T.; Lin, H.; Chen, H.; Liu, J.; Lyu, F.; Ding, Y. Physicochemical properties and microstructure of surimi treated with egg white modified by tea polyphenols. Food Hydrocoll. 2019, 90, 82–89.
- Ramírez, J.; García-Carreño, E.; Morales, O.; Sánchez, A. Inhibition of modori-associated proteinases by legume seed extracts in surimi production. J. Food Sci. 2002, 67, 578–581.
- Luo, Y.; Kuwahara, R.; Kaneniwa, M.; Murata, Y.; Yokoyama, M. Effect of soy protein isolate on gel properties of Alaska pollock and common carp surimi at different setting conditions. J. Sci. Food Agric. 2004, 84, 663–671.
- Luo, Y.; Shen, H.; Pan, D. Gel-forming ability of surimi from grass carp (Ctenopharyngodon idellus): Influence of heat treatment and soy protein isolate. J. Sci. Food Agric. 2006, 86, 687–693.
- Rawdkuen, S.; Benjakul, S. Whey protein concentrate: Autolysis inhibition and effects on the gel properties of surimi prepared from tropical fish. Food Chem. 2008, 106, 1077–1084.
- Liu, L.; Luo, Y.; Song, Y.; Shen, H.; Hong, H. Study on gel properties of silver carp (Hypophthalmichthys molitrix) and whitecroaker (Argyrosomus argentatus) blended surimi at different setting conditions. J. Aquat. Food Prod. Technol. 2013, 22, 36–46.
- Yi, S.; Ji, Y.; Guo, Z.; Zhu, J.; Xu, Y.; Li, X.; Li, J. Gel properties and flavor characteristics of blended anchovy (Engraulis japonicus) mince and silver carp (Hypophthalmichthys molitrix) surimi. RSC Adv. 2020, 10, 6563–6570.
- Gui, P.; Zhang, L.; Hong, H.; Feng, L.; Luo, Y. Gel properties of silver carp (Hypophthalmichthys molitrix) and chicken mixture gels as affected by setting temperatures. Int. J. Food Prop. 2018, 21, 2250–2264.
- Benjakul, S.; Phatcharat, S.; Tammatinna, A.; Visessanguan, W.; Kishimura, H. Improvement of gelling properties of lizardfish mince as influenced by microbial transglutaminase and fish freshness. J. Food Sci. 2008, 73, S239–S246.
- Chanarat, S.;Benjakul, S.; H-Kittikun, A. Comparative study on protein cross-linking and gel enhancing effect of microbial transglutaminase on surimi from different fish. J. Sci. Food Agric. 2012, 92, 844–852.
- Ramírez, J.; Santos, I.; Morales, O.; Morrissey, M.; Vázquez, M. Application of microbial transglutaminase to improve mechanical properties of surimi from silver carp. CYTA-J. Food 2000, 3, 21–28.
- Kaewudom, P.; Benjakul, S.; Kijroongrojana, K. Properties of surimi gel as influenced by fish gelatin and microbial transglutaminase. Food Biosci. 2013, 1, 39–47.
- Singh, A.; Prabowo, F.F.; Benjakul, S.; Pranoto, Y.; Chantakun, K. Combined effect of microbial transglutaminase and ethanoliccoconut husk extract on the gel properties and in-vitro digestibility of spotted golden goatfish (Parupeneus heptacanthus) surimi gel. Food Hydrocoll. 2020, 109, 106107.
- Fang, M.; Xiong, S.; Hu, Y.; Yin, T.; You, J. In vitro pepsin digestion of silver carp (Hypophthalmichthys molitrix) surimi gels after cross-linking by Microbial Transglutaminase (MTGase). Food Hydrocoll. 2019, 95, 152–160.
- Fang, M.; Xiong, S.; Yin, T.; Hu, Y.; Liu, R.; Du, H.; Liu, Y.; You, J. In vivo digestion and absorption characteristics of surimi gels with different degrees of cross-linking induced by transglutaminase (TGase). Food Hydrocoll. 2021, 121, 107007.
- Tahergorabi, R.; Beamer, S.K.; Matak, K.E.; Jaczynski, J. Salt substitution in surimi seafood and its effects on instrumental quality attributes. LWT—Food Sci. Technol. 2012, 48, 175–181.
- Wang, G.; Liu, M.; Cao, L.; Yongsawatdigul, J.; Xiong, S.; Liu, R. Effects of different NaCl concentrations on self-assembly ofsilver carp myosin. Food Biosci. 2018, 24, 1–8.
- Bashir, K.M.I.; Kim, J.S.; An, J.H.;Sohn, J.H.; Choi, J.S. Natural food additives and preservatives for fish-paste products: A review of the past, present, and future states of research. J. Food Qual. 2017, 2017, 9675469.
- Tahergorabi, R.; Jaczynski, J. Physicochemical changes in surimi with salt substitute. Food Chem. 2012, 132, 1281–1286.
- Feng, J.; Cao, A.; Cai, L.; Gong, L.; Wang, J.; Liu, Y.; Zhang, Y.; Li, J. Effects of partial substitution of NaCl on gel properties offish myofibrillar protein during heating treatment mediated by microbial transglutaminase. LWT—Food Sci. Technol. 2018, 93, 1–8.
- Walayat, N.; Xiong, H.; Xiong, Z.; Moreno, H.M.; Nawaz, A.; Niaz, N.; Randhawa, M.A. Role of cryoprotectants in surimi andfactors affecting surimi gel properties: A review. Food Rev. Int. 2020, 1–20.
- Lin, J.; Hong, H.; Zhang, L.; Zhang, C.; Luo, Y. Antioxidant and cryoprotective effects of hydrolysate from gill protein of bighead carp (Hypophthalmichthys nobilis) in preventing denaturation of frozen surimi. Food Chem. 2019, 298, 124868.
- Xie, J.; Yan, Y.; Pan, Q.; Shi, W.; Gan, J.; Lu, Y.; Tao, N.; Wang, X.; Wang, Y.; Xu, C. Effect of frozen time on Ctenopharyngodon idella surimi: With emphasis on protein denaturation by Tri-step spectroscopy. J. Mol. Struct. 2020, 1217, 128421.
- Kong, B.; Guo, Y.; Xia, X.; Liu, Q.; Li, Y.; Chen, H. Cryoprotectants reduce protein oxidation and structure deterioration induced by freeze-thaw cycles in common carp (Cyprinus carpio) surimi. Food Biophys. 2013, 8, 104–111.
- Chen, H.; Kong, B.; Guo, Y.; Xia, X.; Diao, X.; Li, P. The effectiveness of cryoprotectants in inhibiting multiple freeze-thaw-induced functional and rheological changes in the myofibrillar proteins of common carp (Cyprinus carpio) surimi. Food Biophys. 2013, 8, 302–310.
- Liu, Q.; Kong, B.; Han, J.; Chen, Q.; He, X. Effects of superchilling and cryoprotectants on the quality of common carp (Cyprinus carpio) surimi: Microbial growth, oxidation, and physiochemical properties. LWT—Food Sci. Technol. 2014, 57, 165–171.
- Pan, J.; Shen, H.; Luo, Y. Cryoprotective effects of trehalose on grass carp (Ctenopharyngodon idellus) surimi during frozen storage. J. Food Process. Preserv. 2010, 34, 715–727.
- Zhang, L.; Li, Q.; Hong, H.; Luo, Y. Prevention of protein oxidation and enhancement of gel properties of silver carp(Hypophthalmichthys molitrix) surimi by addition of protein hydrolysates derived from surimi processing by-products. Food Chem. 2020, 316, 126343.
- Samaranayaka, A.G.; Li-Chan, E.C. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254.
- Wiriyaphan, C.; Chitsomboon, B.; Roytrakul, S.; Yongsawadigul, J. Isolation and identification of antioxidative peptides from hydrolysate of threadfin bream surimi processing byproduct. J. Funct. Foods 2013, 5, 1654–1664.
- Nikoo, M.; Benjakul, S.; Gavlighi, H.A.; Xu, X.; Regenstein, J.M. Hydrolysates from rainbow trout (Oncorhynchus mykiss) processing by-products: Properties when added to fish mince with different freeze-thaw cycles. Food Biosci. 2019, 30, 100418.
- Sidebottom, C.; Buckley, S.; Pudney, P.; Twigg, S.; Jarman, C.; Holt, C.; Telford, J.; McArthur, A.; Worrall, D.; Hubbard, R. Heat-stable antifreeze protein from grass. Nature 2000, 406, 256.
- Knight, C.A. Adding to the antifreeze agenda. Nature 2000, 406, 249–251.
- Li, X.; Liu, C.; Wang, J.; Li, W.; Lin, B.; Zhu, W.; Xu, Y.; Yi, S.; Mi, H.; Li, J. Tea polyphenols affect oxidative modification and solution stability of myofibrillar protein from grass carp (Ctenopharyngodon idellus). Food Biophys. 2020, 15, 397–408.
- Li, J.; Munir, S.; Yu, X.; Yin, T.; You, J.; Liu, R.; Xiong, S.; Hu, Y. Interaction of myofibrillar proteins and epigallocatechin gallate in the presence of transglutaminase in solutions. Food Funct. 2020, 11, 9560–9572.
- Shi, L.; Wang, X.; Chang, T.; Wang, C.; Yang, H.; Cui, M. Effects of vegetable oils on gel properties of surimi gels. LWT—Food Sci. Technol. 2014, 57, 586–593.
- Chang, T.; Wang, C.; Wang, X.; Shi, L.; Yang, H.; Cui, M. Effects of soybean oil, moisture and setting on the textural and color properties of surimi gels. J. Food Qual. 2015, 38, 53–59.
- Luo, Y.; Kuwahara, R.; Kaneniwa, M.; Murata, Y.; Yokoyama, M. Comparison of gel properties of surimi from Alaska pollockand three freshwater fish species: Effects of thermal processing and protein concentration. J. Food Sci. 2001, 66, 548–554.
- Chan, J.; Gill, T.; Paulson, A. Cross-linking of myosin heavy chains from cod, herring and silver hake during thermal setting. J. Food Sci. 1992, 57, 906–912.
- Yi, S.; Ye, B.; Li, J.; Wang, W.; Li, X. Physicochemical properties, protein conformation, and aggregate morphology of heated myosin from Hypophthalmichthys molitrix and Nemipterus virgatus mixtures. Food Front. 2020, 1, 473–483.
- Riebroy, S.; Benjakul, S.; Visessanguan, W.; Erikson, U.; Rustad, T. Comparative study on acid-induced gelation of myosin from Atlantic cod (Gardus morhua) and burbot (Lota lota). Food Chem. 2008, 109, 42–53.
- Sylvia, S.; Claus, J.; Marriott, N.; Eigel, W. Low-fat, high-moisture frankfurters: Effects of temperature and water during extended mixing. J. Food Sci. 1994, 59, 937–940.
- Bechtel, P.J. Properties of different fish processing by-products from pollock, cod and salmon. J. Food Process. Preserv. 2003, 27, 101–116.
- Robin, J.; Cravedi, J.P.; Hillenweck, A.; Deshayes, C.; Vallod, D. Off flavor characterization and origin in French trout farming. Aquaculture 2006, 260, 128–138.
- Selli, S.; Prost, C.; Serot, T. Odour-active and off-odour components in rainbow trout (Oncorhynchus mykiss) extracts obtained by microwave assisted distillation–solvent extraction. Food Chem. 2009, 114, 317–322.
- Zhang, H.; Wu, D.; Huang, Q.; Liu, Z.; Luo, X.; Xiong, S.; Yin, T. Adsorption kinetics and thermodynamics of yeast β-glucan for off-odor compounds in silver carp mince. Food Chem. 2020, 319, 126232.
- Yamprayoon, J.; Noomhorm, A. Geosmin and off-flavor in Nile tilapia (Oreochromis niloticus). J. Aquatic Food Prod. Technol. 2000, 9, 29–41.
- Zhao, Y.; Yu, J.; Su, M.; An, W.; Yang, M. A fishy odor episode in a north China reservoir: Occurrence, origin, and possible odor causing compounds. J. Environ. Sci. 2013, 25, 2361–2366.
- Chen, D.; Chen, X.; Chen, H.; Cai, B.; Wan, P.; Zhu, X.; Sun, H.; Sun, H.; Pan, J. Identification of odor volatile compounds anddeodorization of Paphia undulata enzymatic hydrolysate. J. Ocean Univ. China 2016, 15, 1101–1110.
- Jin, R.; Meng, R.; Zhang, H.; Yang, X.; Wu, Z. Effects of different deodorising processes on the off-odour compounds and gel properties of common carp surimi. Int. J. Food Sci. Technol. 2018, 53, 2045–2053.
- Xue, C.; You, J.; Zhang, H.; Xiong, S.; Yin, T.; Huang, Q. Capacity of myofibrillar protein to adsorb characteristic fishy-odor compounds: Effects of concentration, temperature, ionic strength, pH and yeast glucan addition. Food Chem. 2021, 363, 130304.
- Anonymous. China Fishery Statistical Yearbook 2021; China Agriculture Press: Beijing, China, 2022; pp. 1–158.
- El-Banna, S.; Atallah, S. Study the role of feed additives in prevention of fish diseases incidence in Oreochromis niloticus and common carp fish and its economic importance. J. Arab. Aquac. Soc. 2009, 4, 121–139.
- Vandeputte, M. Selective breeding of quantitative traits in the common carp (Cyprinus carpio): A review. Aquat. Living Resour. 2003, 16, 399–407.
- Kestemont, P. Different systems of carp production and their impacts on the environment. Aquaculture 1995, 129, 347–372.
