The organic carbamates and carbonates are highly desirable compounds that have found a wide range of applications in drug design, medicinal chemistry, material science, and the polymer industry. The development of new catalytic carbonate and carbamate forming reactions, which employ carbon dioxide as a cheap, green, abundant, and easily available reagent, would thus represent an ideal substitution for existing methods. In this review, the advancements in the catalytic conversion of allylic and propargylic alcohols and amines to corresponding five-membered cyclic carbonates and carbamates are summarized.
1. Introduction
Very few molecules provoke as much controversy as carbon dioxide (CO
2). Since the beginning of the industrial age, the concentration of CO
2 in the Earth’s atmosphere has increased by about 46% [
1]. It is estimated that currently, 93% of anthropogenic CO
2 is generated by fossil fuel combustion [
2], with the prediction that the atmospheric concentration of CO
2 will increase between 730 and 1020 ppm by the end of the century [
3]. The connection between the increasing concentration of CO
2 and ocean acidity, as well as severe and obvious global climate changes, have initiated the trend towards the substantial reduction in fossil fuels and CO
2 emission [
4], moving towards alternative energy sources and a “low-carbon economy”.
The development of new strategies and techniques for carbon capture, utilization, and storage (CCUS) are currently subjects of intense research. However, it is important to note that energy technology deployment is slow [
5], and not all CCUS processes are financially viable or amenable for scaling up [
6]. In addition, with the advancement of CO
2 storage, scientists have developed novel and innovative approaches to direct the chemical and pharmaceutical industries towards a carbon-sustainable chemical synthesis by using CO
2 gas as an abundant carbon source in terms of the concept of green chemistry. This movement remains challenging because of the exceptional thermodynamic stability of CO
2 and thus, the high energetic cost of its fixation. Indeed, the attempts to reduce CO
2 back to a synthetically useful compound might require comparable or more energy than obtained by its production by the combustion of fossil fuel, as CO
2 represents the final product of the carbon oxidation [
7]. Likewise, the kinetic barrier of CO
2 reduction is high. Even though the methanation of CO
2 is an exergonic reaction, as H
2 gas is highly energetically rich, it does not proceed without a metal catalyst, and problems considering H
2 storage should also be addressed. The Sabatier reaction, discovered in 1902, uses an Ni catalyst and H
2 to reduce CO
2 to methane, and this reaction is currently experiencing a revival due to its potential use in long-haul space missions [
8].
2. Cyclic Carbonates
Cyclic carbonates are of great significance in the chemical industry, with a wide range of important applications. They are used as highly polar solvents [
17], electrolytes in lithium batteries [
18], precursors for polycarbonates forming the major class of commercial thermoplastics [
19], and precursors for other organic compounds [
20]. The simplest cyclic carbonate, ethylene carbonate, is synthesized industrially by the reaction of ethylene oxide with CO
2.
Five-Membered Cyclic Carbonates from Allylic Alcohols
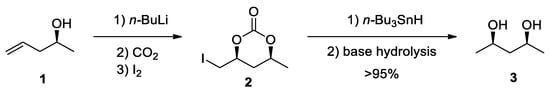
In 1981, Cardillo et al. demonstrated the first synthesis of 5-iodomethyl cyclic carbonates of type 2 by employing CO
2. The synthesis involved the engagement of a strong base (
n-BuLi) in a CO
2-saturated THF solution of iodine [
21]. This method showed a high degree of regioselectivity and stereoselectivity. For example, the allylic alcohols always led to the formation of 5-membered cyclic carbonates, while homoallylic alcohols yielded 6-membered rings. The high stereoselectivity of the carbonation of pent-4-en-2-ol (
1) was proven by the almost exclusive formation of
syn-diol 3 (>95%) produced by the treatment of the 5-iodomethyl cyclic carbonate
2 with Bu
3SnH (to convert C–I into C–H) under basic conditions (
Scheme 1). The mechanism of the reaction was proposed to include an iodonium ion as the key intermediate, which opens by nucleophilic attack of the in situ formed carbonate anion.
Scheme 1. Cardillo’s synthesis of a 5-iodomethyl cyclic carbonate and its stereoselectivity [
21].
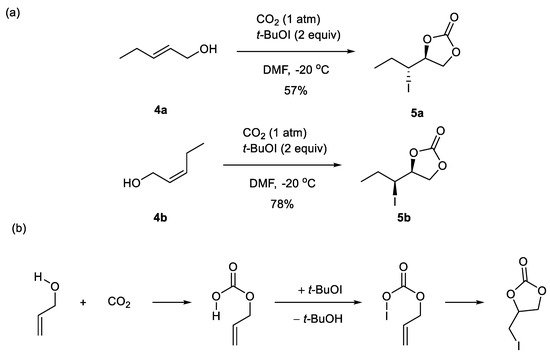
In 2010, Minakata et al. reported an improved version of this reaction by using
t-BuOI, a powerful iodinating agent, which was previously proven to be efficient for the cyclization of the unsaturated amines (
Scheme 2a) [
22]. In situ formed
t-BuOI, by the reaction of
t-BuOCl with NaI, gave higher yields than other iodine sources, such as
N-iodosuccinimide and I
2. In addition, reaction conditions were simpler, as the reaction can be conducted under ambient pressure, avoiding the saturation of the solution with CO
2. This method was also more general and gave higher yields with allylic, homoallylic, and propargylic alcohols [
19]. Interestingly,
E-hex-2-en-1-ol (
4a) gave the single
anti-diastereomer of 4-(1-iodopropyl)-1,3-dioxolan-2-one (
5a), whereas the
syn-carbonate
5b was exclusively obtained from
4b (
Scheme 2a). Although the products
anti- and
syn-
5 were formed in only 57% and 78% yields, respectively, the reaction was categorized as stereospecific. Further studies showed that the optimal reaction conditions are based on the use of
t-BuOI (2 equiv) in THF at −20 °C. Under these conditions, the unsubstituted propargylic alcohol provided
E-isomer in a 92% yield after 3 h. The mechanism is assumed to proceed through a hydrogen-iodine exchange at the carbonate oxygen junction, followed by cyclization (
Scheme 2b).
Scheme 2. (
a) The stereoselectivity of Minakata’s synthesis of 5-iodomethyl cyclic carbonates; (
b) the proposed mechanism of Minakata’s synthesis of 5-iodomethyl cyclic carbonates. The nucleophilic attack of the alcohol group on CO
2 is followed by hydrogen–iodine exchange. The now electrophilic oxygen is attacked by the double bond, leading to cyclisation [
22].
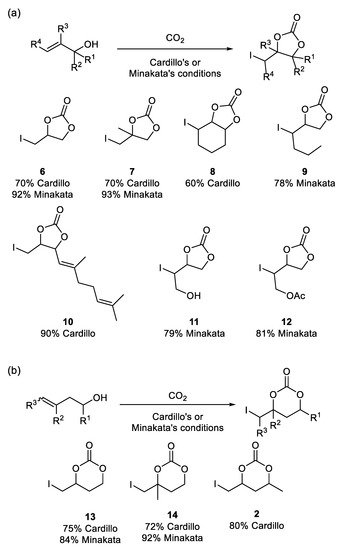
A comparison of Cardillo’s and Minakata’s methods for carboxylative cyclization of allylic (a) and homoallylic (b) alcohols is shown in
Scheme 3. In all cases, Minakata’s conditions led to the formation of pentacyclic and hexacyclic carbonates in higher yields.
Scheme 3. A comparison of Cardillo’s and Minakata’s carboxylative cyclization of allylic (
a) and homoallylic alcohols (
b). Conditions: Cardillo—(1 equiv
nBuLi in THF added to alcohol, then CO
2 bubbled and 2 equiv I
2 added, stirred for 14 h at rt) [
21], and Minakata—(2 equiv
t-BuOCl added to 1 equiv NaI and alcohol under CO
2 atmosphere, stirred for 3–72 h at −20 °C) [
22].
3. Cyclic Carbamates
Because of its great chemical and proteolytic stability, as well as the ability to easily penetrate into cells, the carbamate motif is a very important peptide bond surrogate in the drug development process [
49]. Vacondio et al. reviewed and examined medicinal applications of carbamates, concluding that cyclic carbamates are stable and prone to metabolic hydrolysis [
50]. Thus, cyclic carbamates have become a subject of increased research for pharmaceuticals.
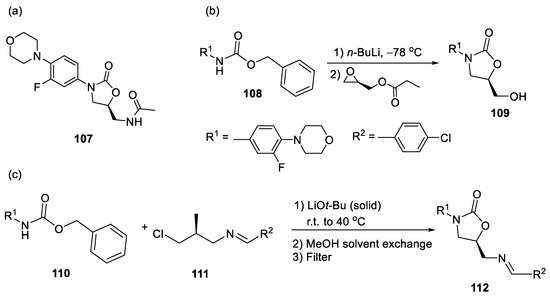
For example, a very important antibiotic used to treat infections by various Gram-positive bacteria is Linezolid
107, a drug which contains the 5-membered cyclic carbamate (2-oxazolidinone) motif (
Scheme 26a). The first-generation synthesis of linezolid reported by Pfizer employed a glycidyl ester and a strong base (
n-BuLi) at a low temperature to obtain the cyclic carbamate moiety (
Scheme 26b) [
51]. The “greener” second generation synthesis used imine derivative
111 under milder conditions (
Scheme 26c). However, the latter method still employs a strong base and generates a lot of waste. Thus, the development of new reactions and reaction conditions with high atom economy, such as the carboxylative cyclizations of unsaturated amines and CO
2, is very desirable and could be directly applied in the green and highly valuable industrial synthesis of linezolid and its derivatives.
Scheme 26. (
a) Linezolid
107; (
b) formation of the carbamate moiety in the first synthesis of linezolid [
51] and (
c) in the second-generation synthesis [
52].
4. Conclusions
The utilization of CO2 to produce value-added compounds is currently one of the most researched topics in green chemistry. This paper summarized the progress in the synthesis of 5-membered cyclic carbonates and carbamates from the corresponding unsaturated alcohols and amines. These are valuable compounds in the chemical and pharmaceutical industry. Despite significant advancement in the methodologies of their production, there are still significant challenges associated with the CO2 fixation process, such as the lack of environmentally friendly or metal-free catalysts, as well as catalyst reusability. Ongoing research in multiple directions aims to address these issues.
This entry is adapted from the peer-reviewed paper 10.3390/catal12050547