Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Organic
|
Chemistry, Medicinal
Organoselenium (OSe) compounds have recently gained considerable interest as a potential class of organic motifs due to their outstanding applications in synthetic organic and medicinal chemistry and their possible properties in materials science. These are attributed to the exceptional properties of the selenium (Se) element.
- polymerization
- organoselenium
- one-pot multicomponent
1. Introduction
Organoselenium (OSe) compounds have recently gained considerable interest as a potential class of organic motifs due to their outstanding applications in synthetic organic and medicinal chemistry and their possible properties in materials science [1,2,3,4]. These are attributed to the exceptional properties of the selenium (Se) element. The latter is a non-metal present in almost living organisms as part of selenoproteins (e.g., thioredoxin reductases and glutathione peroxidase antioxidants enzymes) [5,6,7,8,9]. Accordingly, Se is crucial for protecting human cells from oxidative damage and the immune system’s normal function [10,11,12]. Compared to sulfur (S), Se has a larger atomic radius (S: 1.02 Å vs. Se: 1.17 Å), lower electronegativity (S: 2.58 vs. Se: 2.55), higher polarizability (S: 2.9 Å vs. Se: 3.8 Å), and therefore Se is a likely better nucleophile than the S [5,8,13]. Accordingly, OSe compounds are known for their ability to react with O2-free radicals and thus prevent the progression of oxidative stress-related diseases [3,6,10,14]. Furthermore, OSe compounds were also used in material science due to their semiconductor potential and therefore used in sodium-ion batteries, solar cells, and H2 evolution catalysts [2,15,16,17]. Moreover, the Se center is present in many naturally occurring and bioactive interesting OSe compounds (Figure 1), such as the selenoaminoacids (e.g., selenocysteine (I), selenomethionine (II), and selenocystine (III)) [18,19,20]. Furthermore, ebselen (IV) is the most investigated Se compound with exciting GPX-like activity and has recently reached clinical phase III trials as a possible drug for Meniere’s disease [11,21,22] (Figure 1). Moreover, ethaselen (V) entered trial phase II for non-small lung cancer treatment [23,24,25,26]. On the other hand, different OSe compounds have also manifested efficient catalytic activity for various organic reactions, such as the palladium-based OSe complex VI for the Heck reaction (Figure 1) [27].
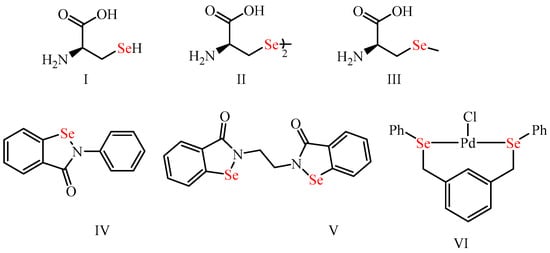
Figure 1. Organoselenium compounds (I–VI) with potential biological activities and diverse applications.
Given the exciting activities and the diverse applications of the OSe compounds, sustainable and efficient approaches for their preparation are in high demand. The synthesis of OSe compounds depends on their chemical structures (e.g., selenides, selenocyanates, diselenides) [26,28,29]. Standard methods include direct selenylation via reaction with proper Se reagents such as Na2Se2 and KSeCN. On the other hand, indirect selenylations include rearrangement of Se-containing precursors (e.g., isoselenocyanates) or multistep synthetic procedures using elemental Se together with organolithium or Grignard reagents and [2,13,24,30,31,32,33,34,35]. Despite being efficient, these classical strategies are relatively limited due to the challenges associated with the complicated synthetic procedure, regioselectivity, or harsh reaction conditions issues. Recently, various alternative reactions were developed as efficient and milder synthetic protocols within combinatorial chemistry (CC) [36,37,38,39,40,41,42,43]. The latter has emerged as a robust tool in medicinal chemistry [44,45]. It is now widely and consecutive covalent bonds formed between different building blocks [46]. Concurrently, drug candidates are discovered and selected by the screening of small molecule libraries for particular biological targets [47]. Despite the various strategies used in CC, multicomponent reactions (MCRs) are amongst the most investigated techniques for the efficient synthesis of chemical libraries [43,45]. MCRs have been known for over a century. They include generating skeletally diverse and complex molecular entities from more than two starting materials by straightforward chemical transformations employing comparatively mild conditions [37,48,49,50].
From an economical step and atom viewpoint, the MCR one-pot strategy would offer more robust access to OSe compounds. Though the MCRs have emerged as an efficient tool for constructing C-S bonds, similar approaches for synthesizing C-Se bonds have recently attracted more attention.
2. Metal-Catalyzed Synthesis of the OSe Compounds
In 2013, de Oliveira et al. reported the one-pot Cu-catalyzed (CuCl) synthesis of OSe propargylamines in excellent yields (up to 95%) via A3-coupling of trimethylsilyl Se-acetylene, p-methoxybenzaldehyde, and piperidine catalyzed in DCM as the solvent and in the presence of succinic acid additive at 50 °C (Scheme 1) [51].
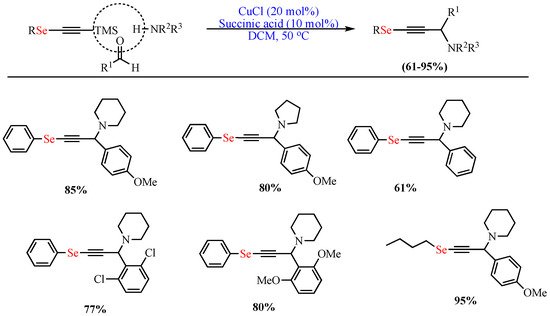
Scheme 1. Synthesis of OSe propargylamines.
In 2017, Liu et al. reported the synthesis of highly functionalized isoselenoureas through the Cu-catalyzed (CuI) 1,10-phenanthroline-promoted MCR of elemental Se, aryl iodides, isocyanides, and amines using Cs2CO3 as the base and THF as the solvent at 70 °C (Scheme 2) [52].
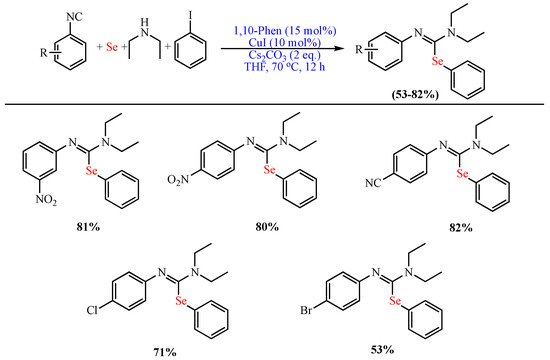
Scheme 2. Synthesis of functionalized isoselenoureas.
In 2018, Aquino et al. synthesized (arylselanyl)-alkyl-1,2,3-triazolo-1,3,6-triazonines via CuI-catalyzed MCR of 2-azidobenzaldehyde, 1,2-diaminobenzene, and various arylchalcogenyl alkynes in dioxane at 100 °C. The reaction included a wide variety of arylchalcogenyl alkynes (Scheme 3) [53].
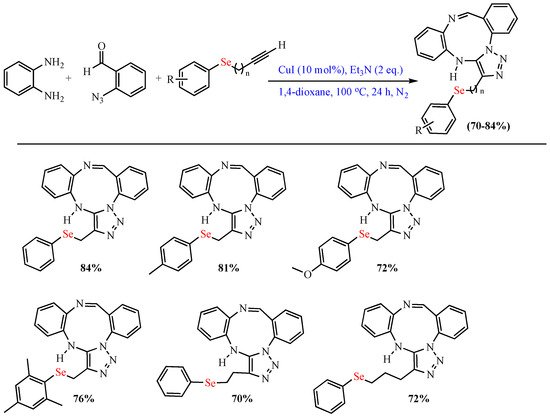
Scheme 3. Synthesis of (arylselanyl)-alkyl-1,2,3-triazolo-1,3,6-triazonines.
Zhang et al. reported the sequential multistep Cu-catalyzed (CuI) assembly of 5-selenotriazoles via the one-pot reaction of elemental Se, azide, alkyl halide, and alkyne. The selenylating agent and Cu(I) triazolides were generated in situ, and the reaction proceeded under mild conditions using readily available substrates with broad variety scope in high regioselectivity (Scheme 4) [54].
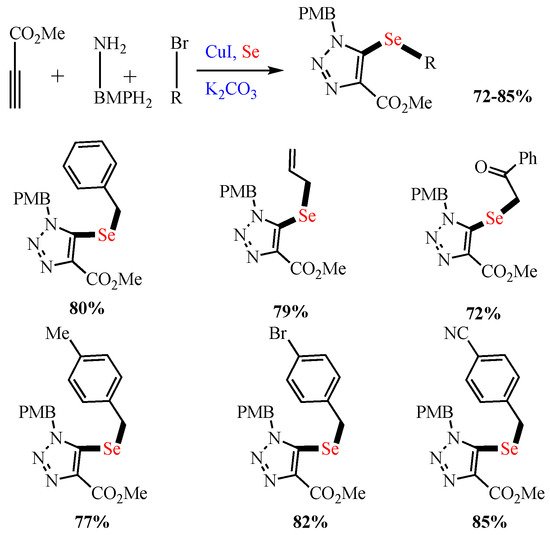
Scheme 4. Synthesis of 5-selenotriazoles.
In 2019, Gao et al. reported the Cu-catalyzed (Cu(OAc)2) cross-coupling oxidative aminoarylselenation of maleimides using elemental Se, aryl iodides, and secondary amines. This reaction enabled the bifunctionalization of alkenes via the simultaneous one-pot construction of the C−N bond and C−Se bonds (Scheme 5) [55].
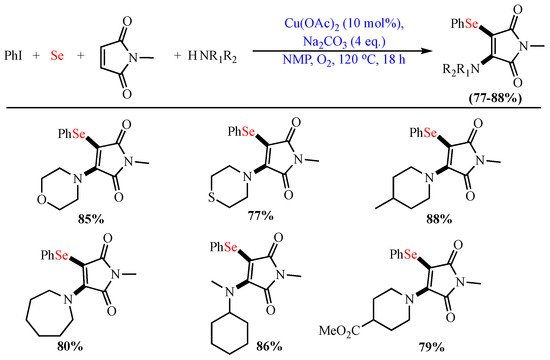
Scheme 5. Aminoarylselenation of maleimides.
Recently, Begini et al. developed 4-arylselanyl-1H-1,2,3-triazoles from the one-pot Cu-catalyzed (Cu(OAc)2) reaction of selanylalkynylcarbinols and aryl azides in the presence of sodium ascorbate (10 mol%) in H2O and THF (1:1) mixed solvent at 50 °C (Scheme 6) [56].
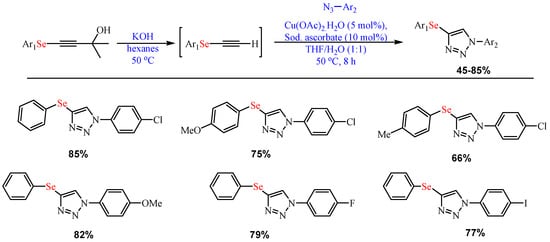
Scheme 6. Synthesis of 4-arylselanyl-1H-1,2,3-triazoles.
In 2021, Rather et al. disclosed the Cu-catalyzed (CuBr) synthesis of 3-((arylethynyl)selanyl)-1H-indoles in good yield (up to 83%) from elemental Se phenylacetylene and indole using K2CO3 as the base and DMSO as the solvent at 100 °C. The strategy tolerates different indoles and phenylacetylene motifs and can be expanded to a gram scale without any difficulties (Scheme 7) [57].
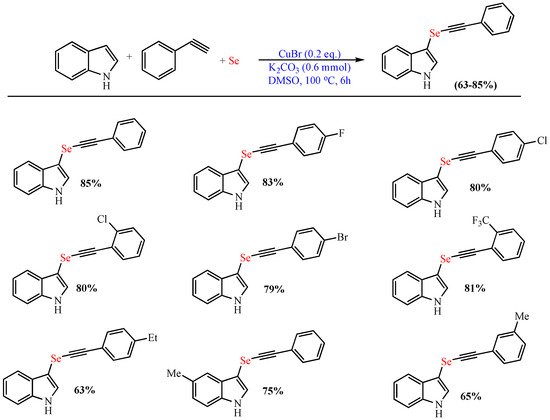
Scheme 7. Synthesis of 3-((arylethynyl)selanyl)-1H-indoles.
Furthermore, Lara et al. reported the synthesis of (Z)-1,2-bis-arylselanyl alkenes by the one-pot reaction of terminal alkynes with diaryl diselenides using KF/Al2O3 and PEG-400 as a solvent in good yields. Interestingly, the reaction time was reduced from 6 h under conventional conditions to 30 min using microwave irradiation (Scheme 8) [58].
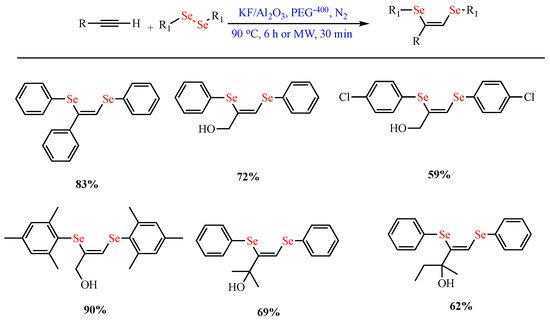
Scheme 8. Synthesis of (Z)-1,2-bis-arylselanyl alkenes.
De Oliveira et al. reported the ytterbium(III)-catalyzed (Yb(OTf)3) synthesis of 2,4- disubstituted Se-quinoline derivatives via Povarov MCR between ethyl glyoxylate, p-anisidine, and ethynyl(phenyl)selane in CH3CN as the solvent at 80 °C and in moderate yields (up to 69%) (Scheme 9) [59].
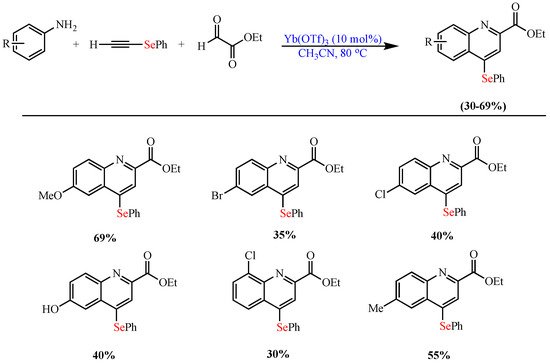
Scheme 9. Synthesis of 2,4- disubstituted Se-quinoline derivatives.
Moreover, Sakai et al. reported the indium-catalyzed (InCl3/PhSiH3) one-pot synthesis of selenolactones from elemental Se and lactones using o-dichlorobenzene as the solvent at 120 °C for 24 h (Scheme 10) [60].
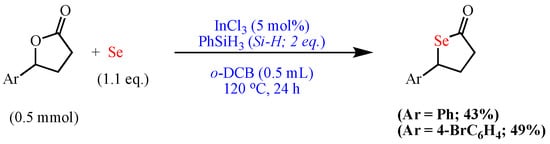
Scheme 10. Synthesis of selenolactones.
Recently, Attia et al. reported the one-pot synthesis of seleno [2,3-b]pyridine derivatives using Ag/AgCl nanoparticles under visible light irradiation. The reaction was carried out under mild conditions using visible light as the energy source, Ag/AgCl- nanoparticles, and EtOH as the solvent. It is worth noting that the Ag/AgCl- nanoparticles showed high catalytic activity and reusability potential up to five cycles in 94–91% isolated yields (Scheme 11) [61].
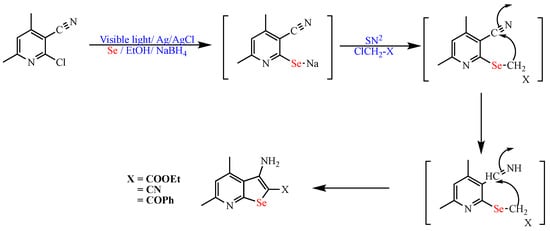
Scheme 11. Synthesis of seleno [2,3-b]pyridine derivatives.
Recently, the same group by Abdel-Hafez et al. reported the synthesis of selenopyridine and quinoline derivatives in excellent yields (up to 90%) and selectivity using Co3O4 nanoparticles heterogeneous catalyst under microwave irradiation and water as the solvent (Scheme 12) [62].
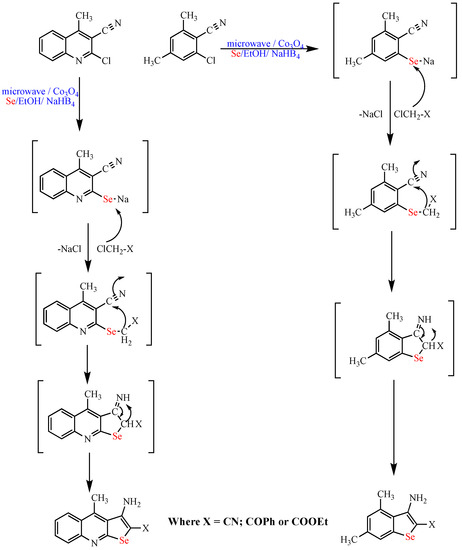
Scheme 12. Synthesis of selenopyridine and quinoline derivatives.
This entry is adapted from the peer-reviewed paper 10.3390/polym14112208
This entry is offline, you can click here to edit this entry!
