Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
免疫疗法研究通常集中在CD8 T细胞上,因为它们具有消除肿瘤细胞的能力。然而,CD4 T细胞之所以引起现场的关注,是因为它们不仅对促进CD8 T细胞功能、防止CD8 T细胞耗竭或诱导CD8 T细胞记忆至关重要,而且能够直接或间接地杀死肿瘤细胞。+++++
- immunotherapy
- hyperprogressive disease
- immune cells
1. Regulatory T (Treg) Cells
In addition to effector cells, the T lymphocyte family includes an immunomodulatory subgroup called Treg cells, whose role is to negatively regulate other immune cells, prevent the overactivation of the immune response, and play a role in a wide range of diseases, such as allergies, chronic infections, and parasitic infections [51]. However, the presence of Treg cells is disadvantageous to hosts with tumors because they limit an effective antitumor immune response. Kamada et al. [52] reported that the proportions of effector regulatory T (eTreg) cells/CD8 T cells, Ki67 Treg cells/Ki67CD8 T cells, and Ki67 Treg cells decreased significantly in non-HPD patients after treatment with anti-PD-1 antibodies, while these proportions in HPD patients remained stable or even increased slightly. This finding suggested that if the number of CD8 T cells is insufficient to overcome Treg cells, the possibility of HPD development is greatly increased. Furthermore, Treg cells have also been shown to express immune checkpoints, such as PD-1; thus, Treg cells can also be targeted by anti-PD-1 agents [53]. Researchers have observed that knocking out PD-1 in Treg cells or blocking PD-1 with monoclonal antibodies (mAbs) caused Treg cells to gain a stronger proliferative ability and a stronger immunosuppression ability, thus leading to a stronger ability to promote tumor growth. This finding suggested that PD-1 Treg cells play a key role in anti-PD-1 treatment-mediated HPD in advanced gastric cancer. In addition, Ratner et al. [37,54] demonstrated that nivolumab led to rapid progression in patients with adult T-cell leukemia/lymphoma (ATLL). They identified a novel relationship between tumor-resident Tregs and ATLL cells and revealed the tumor suppressive effect of PD-1 in ATLL.+++++++
Furthermore, in Treg cells treated with PD-1 blockade, the expression of immune checkpoints is upregulated, and the immunosuppressive function is enhanced. Thus, the antitumor immunity of some patients after anti-PD-1 treatment is not enhanced but greatly weakened, which leads to the occurrence of HPD. Interestingly, CTLA-4 was found to be strongly expressed in Treg cells [55]. Anti-CTLA-4 treatment increased the presence of Ki67 Treg cells [52]. Furthermore, the combination of anti-CTLA-4 antibodies and anti-PD-1 antibodies was associated with a lower incidence of HPD than other ICI combinations, and CTLA-4, OX-40, or CCR4-targeted therapy might be a strategy for preventing HPD through Treg cell consumption [56]. In addition, selective PD-1/PD-L1 inhibition may lead to tumor immune evasion and accelerate tumor growth by increasing the number of Treg cells infiltrating and circulating in the tumor [57].+
2. Other Subsets of CD4 T Cells+
CD4CD28+− T cells are a cell subpopulation with unique biological effects that frequently appear in some autoimmune diseases [58]. Due to their lack of CD28, which is necessary for a cell-specific immune response and the most important costimulatory molecule on the T-cell surface, these unique cells not only have abnormal immune function but also have the characteristics of autoreactivity, massive expansion, and a long lifespan [59]. Arasanz et al. [60] found that CD4CD28+− T cells in the peripheral blood of lung cancer patients with HPD were amplified after PD-1 treatment, and high tumor growth dynamics scores were associated with the presence of CD4CD28+− T-cell subsets in patients with HPD.
In addition, Zappasodi et al. [61] observed in melanoma mice that a subset of CD4Foxp3+−PD-1high T cells can perform immunosuppressive functions similar to those of Treg cells, but their RNA expression levels may be more similar to those of follicular helper T (Tfh) cells. Interestingly, while anti-PD-1 treatment reduced the numbers of these cells, anti-CTLA4 treatment increased their intratumoral abundance. This result suggests that this cell subpopulation could also respond to ICB, proliferate under anti-CTLA-4 treatment, and acquire negative regulatory immune properties, which might contribute to the development of HPD (Figure 1).
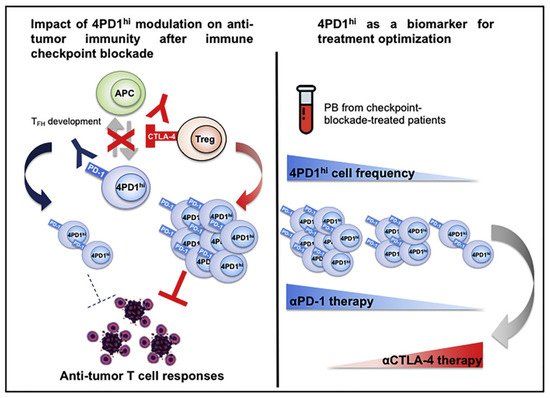
图 1.Zappasodi等人说明了4PD1的功能你好细胞 (PD-1CD4Foxp3++−T细胞)并观察到这些细胞在肿瘤内积累作为肿瘤负担的功能。经参考文献[61]许可转载。2018年,爱思唯尔。
3. CD4 T细胞耗尽+
HPD的另一个潜在机制是耗尽的CD4 T细胞与抗PD-1治疗之间的相关性。目前对CD4 T细胞衰竭的认识显然是不够的。然而,CD4 T细胞衰竭对增殖,细胞因子产生,B细胞帮助和CD8效应器功能的负面影响已有报道。此外,耗尽的CD4 T细胞上调免疫调节蛋白,如TIM3和PD-1,与在耗尽的CD8 T细胞中观察到的表型相似[62]。与非HPD患者不同,HPD患者在初始给予抗PD-1/PD-L1抗体后,外周耗尽的记忆CD4 T细胞表现出异常扩张[60]。Arasanz等人[60]监测了用抗PD-1 / PD-L1抗体治疗的NSCLC患者的外周血单核细胞(PBMC),并且在HPD患者中观察到外周耗尽的CD4 T细胞增殖。他们提出外设CD28的快速扩展++++++++−CD4 T 细胞是 ICP 诱导的 NSCLC 中 HPD 的早期鉴别特征。虽然耗尽的CD4 T细胞的作用尚不完全清楚,但这些研究提供了重要的证据,证明这些细胞也可能有助于HPD的进展。++
4. IFN-γ
虽然 IFN - γ被认为是抗肿瘤免疫的关键因素 [ 63 , 64 ] ,但 Xiao 等人 [ 65 ] 证明 IFN - γ可以通过增强 Th17 相关的炎症反应来促进免疫逃逸和状瘤的发展。因此,IFN-γ可以根据病理背景和选择性应激水平促进抗肿瘤免疫或免疫逃逸[66]。Sakai等人[67]报道,在结核分枝杆菌感染的小鼠模型中,PD-1−导致CD4 + T细胞广泛渗透到肺实质中并产生大量IFN-γ,导致疾病进展迅速,与野生型小鼠相比。此外,编码IFN-γ信号通路成分的基因突变,如IFN-γ受体和JAK1/2,已被确定为抗PD-1/PD-L1和抗CTLA-4抗体耐药的潜在机制[64,68]。Champiat等人[40]指出,ICB下TME中的T细胞行为可能受到影响IFN-γ信号通路的突变的影响,特别是JAK1 / 2中的突变。JAK1/2突变已被证明与ICIs的原发性耐药性相关[69]。此外,已有报道,IFN-γ诱导的干扰素调节因子8(IRF-8)与其启动子结合并诱导MDM2过表达[70,71]。MDM2是一种参与p53降解和抑制的蛋白质,其扩增在HPD患者中经常观察到[70]。
This entry is adapted from the peer-reviewed paper 10.3390/cells11111758
This entry is offline, you can click here to edit this entry!
