Neurodegenerative disorders including Parkinson’s disease (PD), Huntington’s disease (HD) and the most frequent, Alzheimer’s disease (AD), represent one of the most urgent medical needs worldwide. Despite a significantly developed understanding of disease development and pathology, treatments that stop AD progression are not yet available. The approval of sodium oligomannate (GV-971) for AD treatment in China emphasized the potential value of natural products for the treatment of neurodegenerative disorders. Many current clinical studies include the administration of a natural compound as a single and combination treatment. The most prominent mechanisms of action are anti-inflammatory and anti-oxidative activities, thus preserving cellular survival.
- Alzheimer’s disease
- neurodegeneration
- drug development
- clinical studies
1. Introduction
| Agent | Mechanism of Action | Therapeutic Purpose | Trial Identifier and Status | Phase |
|---|---|---|---|---|
| Huperzine A | AChE inhibitor, inhibition of Aβ | improve memory | Not yet recruiting NCT02931136 |
IV |
| Sodium oligomannate (GV-971) |
neuroinflammation modulators, microbiome modulators, amyloid beta-protein inhibitors; reconditioning the dysbiosis of gut microbiota, preventing peripheral immune cells from invading the brain, inhibiting the inflammatory response in the brain targeting protein folding errors in the brain tissue |
improve the cognitive function of patients with mild to moderate AD | Recruiting NCT05058040 |
IV |
| Sodium oligomannte capsules (GV-971) |
neuroinflammation modulators, microbiome modulators, amyloid beta-protein inhibitors; reconditioning the dysbiosis of gut microbiota, preventing peripheral immune cells from invading the brain, inhibiting the inflammatory response in the brain targeting protein folding errors in the brain tissue |
improve the cognitive function of patients with mild to moderate AD | Recruiting NCT05181475 |
IV |
| Ginkgo biloba | metabolism and bioenergetics; plant extract with antioxidant properties | Improve brain blood flow and mitochondrial function (cognitive enhancer) | Recruiting NCT03090516 |
III |
| Sodium oligomannate (GV-971) |
reconditioning the dysbiosis of gut microbiota, preventing peripheral immune cells from invading the brain, inhibiting the inflammatory response in the brain targeting protein folding errors in the brain tissue | improve the cognitive function of patients with mild to moderate AD; evaluate safety, tolerability and efficacy of GV-971 | Recruiting NCT04520412 |
III |
| Curcumin + aerobic yoga | herb with antioxidant and anti-inflammatory properties | decrease inflammation and oxidation related neurotoxicity | active, not recruiting NCT01811381 |
II |
| Elderberry Juice | rich in anthocyanins, has anti-inflammatory and antioxidant activity | improve mitochondrial function | completed NCT02414607 |
II |
| Grape powder | antioxidant, anti-inflammatory and anticarcinogenic | improves cognitive performance preservation of metabolism in brain regions important to cognitive function | recruiting NCT03361410 |
II |
| Icosapent ethyl (IPE) | synaptic plasticity, neuroprotection; purified from of the omega-3 fatty acid EPA | improve synaptic function; reduce inflammation | recruiting NCT02719327 |
II |
| Meganatrual-Az Grapeseed Extract | polyphenolic extract with antioxidant properties | anti-oligomerization agent; prevents aggregation of amyloid and tau | recruiting NCT02033941 |
II |
| Omega-3 PUFA | fish oil concentrate standardized to long chain in n-3 PUFA content | reduces inflammation and glial activation; enhances amyloid removal; protect small blood vessels | active, not recruiting NCT01953705 |
II |
| Rapamycin | anti-inflammatory, antineoplastic; macrolide compound from Streptomyces hygroscopicus | selectively blocks the transcriptional activation of cytokines | recruiting NCT04629495 |
II |
| Rifaximin | inflammation, infection and immunity; antibiotic | reduce proinflammatory cytokines secreted by harmful gut bacteria | completed NCT03856359 |
II |
| Tacrolimus | tau proteins; macrolide from culture broth of a strain of Streptomyces tsukubaensis | reduce pathological changes of tau proteins | withdrawn NCT04263519 |
II |
| THC-free CBD Oil | anti-oxidant and anti-inflammatory; cannabinoids | behavioural and psychological symptoms of dementia (BPSD) decrease with use of cannabinoids | recruiting NCT04436081 |
II |
| VGH-AD1 | undisclosed; traditional Chinese herbal medicine | undisclosed (cognitive enhancer) | not yet recruiting NCT04249869 |
II |
| Yangxue Qingnao pills | blood circulation; traditional Chinese medicine, composed of Angelicae Sinensis Radix, Chuanxiong Rhizoma, Paeoniae Radix Alba, Rhemannia glutinosa, Uncaria macrophylla Wall, Caulis spatholobi, Spica Prunellae, Catsia tora Linn, Mater Margarita, Corydalis ambigua and Asarum sieboldii | improve cerebral blood flow and brain nourishment | not yet recruiting NCT04780399 |
II |
| BDPP (bioactive dietary polyphenol preparation) | metabolism and bioenergetics, amyloid; combination of grape seed polyphenolic extract and resveratrol | prevents amyloid and tau aggregation | recruiting NCT02502253 |
I |
| Pomace olive oil | prevent inflammation; lipophilic minor components | consumption of olive oil reduces activation of microglia by TRL (triglyceride-rich lipoproteins) | completed NCT04559828 |
not applicable |
| Extra virgin olive oil “Coratina” | anti-amyloid; biophenol | improve cerebral performance | not yet recruiting NCT04229186 |
not applicable |
2. Natural Products from Non-Algal Sources
2.1. Esterase Inhibitors
| Name | Structure | Source | Characteristics | Ref. |
|---|---|---|---|---|
| galantamine | 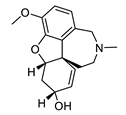 |
Galanthus nivalis | reversible, competitive AChE inhibitor, allosteric modulator of nicotinic acetylcholine receptors, modulates α4β2 and α7 nicotinic receptors | [40][41][42][43] |
| huperzine A | 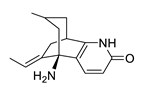 |
Huperzia serrata | specific and reversible AChE inhibitor, protects cells against hydrogen peroxide, β-amyloid toxicity, glutamate, ischemia and staurosporine-induced cytotoxicity and apoptosis | [45][46][47][48][49] |
| physostigmine | 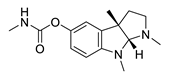 |
Physostigma venenosum, Streptomyces pseudogriseolus | AChE inhibitor | [50] |
| tolserine | 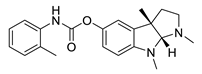 |
Physostigmine derivative | AChE inhibitor | [51] |
| eseroline | 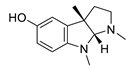 |
Physostigmine derivative | AChE inhibitor | [51] |
| phenserine | 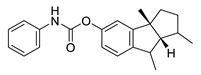 |
Physostigmine derivative | AChE inhibitor | [51] |
2.2. Plant Natural Products with Antioxidant and Anti-Inflammatory Efficacy
| Structure | Ginsenoside | R1 | R2 | R3 | |
|---|---|---|---|---|---|
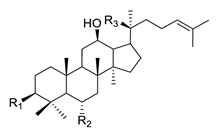 |
PPD-type | Rb1 | -O-Glc-Glc | -H | -O-Glc-Glc |
| Rb2 | -O-Glc-Glc | -H | -O-Glc-Ara(p) | ||
| Rc | -O-Glc-Glc | -H | -O-Glc-Ara(f) | ||
| Rd | -O-Glc-Glc | -H | -O-Glc | ||
| Rg3 | -O-Glc-Glc | -H | -OH | ||
| F2 | -O-Glc | -H | -O-Glc | ||
| Rh2 | -O-Glc | -H | -OH | ||
| Compound K | -OH | -H | -O-Glc | ||
| PPD | -OH | -H | -OH | ||
| PPT-type | Re | -OH | -O-Glc-Rha | -O-Glc | |
| Rf | -OH | -O-Glc-Glc | -OH | ||
| Rg1 | -OH | -O-Glc | -O-Glc | ||
| Rg2 | -OH | -O-Glc-Rha | -OH | ||
| Rh1 | -OH | -O-Glc | -OH | ||
| F1 | -OH | -OH | -O-Glc | ||
| PPT | -OH | -OH | -OH | ||
3. Neuroprotective Algal Metabolites
3.1. Carbohydrates
| Name | Structure | Source | Characteristics | Ref. |
|---|---|---|---|---|
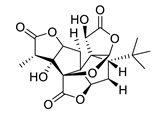
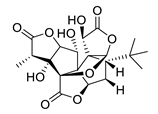
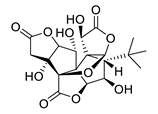
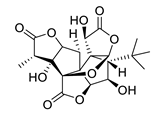
| GV971 (Sodium oligomannate) |
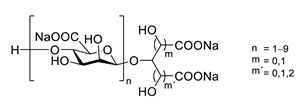 |
marine brown algae | might act via decreasing neuroinflammation by remodeling gut microbiota and balancing the amino acid metabolism, especially phenylalanine and isoleucine | [87] |
| porphyran | 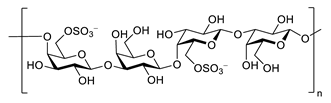 |
Porphyra yezoensis | superoxide anion and hydroxyl radical scavenging activity | [90] |
| floridoside | 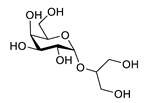 |
Laurencia undulata | anti-inflammatory activity, inhibits the production of NO and ROS, downregulates iNOS and COX-2 | [91] |
| fucoidan | 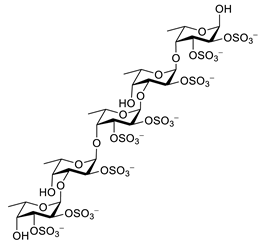 |
Ascophyllum nodosum | inhibits ROS and TNF-α release, reduces NO, PGE2, COX-2, iNOS, MCP-1, TNF-α and IL-1β | [92][93] |
| κ-carrageenan | 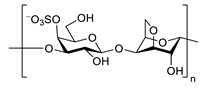 |
inhibits TNF-α secretion | [94] |
This entry is adapted from the peer-reviewed paper 10.3390/biom12050694
References
- Rostagno, A.; Holton, J.L.; Lashley, T.; Revesz, T.; Ghiso, J. Cerebral amyloidosis: Amyloid subunits, mutants and phenotypes. Cell. Mol. Life Sci. 2009, 67, 581–600.
- Sipe, J.D.; Benson, M.D.; Buxbaum, J.N.; Ikeda, S.-I.; Merlini, G.; Saraiva, M.J.; Westermark, P. Amyloid fibril protein nomenclature: 2010 recommendations from the nomenclature committee of the International Society of Amyloidosis. Amyloid 2010, 17, 101–104.
- Hazenberg, B.P. Amyloidosis: A Clinical Overview. Rheum. Dis. Clin. N. Am. 2013, 39, 323–345.
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259.
- Thal, D.R.; Rüb, U.; Orantes, M.; Braak, H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 2002, 58, 1791–1800.
- Hadjichrysanthou, C.; Evans, S.; Bajaj, S.; Siakallis, L.C.; McRae-McKee, K.; de Wolf, F.; Anderson, R.M.; Initiative, T.A.D.N. The dynamics of biomarkers across the clinical spectrum of Alzheimer’s disease. Alzheimer’s Res. Ther. 2020, 12, 1102.
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185.
- Uddin, S.; Kabir, T.; Rahman, S.; Behl, T.; Jeandet, P.; Ashraf, G.M.; Najda, A.; Bin-Jumah, M.N.; El-Seedi, H.R.; Abdel-Daim, M.M. Revisiting the Amyloid Cascade Hypothesis: From Anti-Aβ Therapeutics to Auspicious New Ways for Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 5858.
- Cummings, J.; Lee, G.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s disease drug development pipeline. Alzheimer’s Dementia Transl. Res. Clin. Interv. 2021, 7, e12179.
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339.
- Lichtenthaler, S.F.; Tschirner, S.K.; Steiner, H. Secretases in Alzheimer’s disease: Novel insights into proteolysis of APP and TREM. Curr. Opin. Neurobiol. 2021, 72, 101–110.
- Imbimbo, B.P.; Watling, M. Investigational BACE inhibitors for the treatment of Alzheimer’s disease. Expert Opin. Investig. Drugs 2019, 28, 967–975.
- Luo, J.E.; Li, Y.-M. Turning the tide on Alzheimer’s disease: Modulation of γ-secretase. Cell Biosci. 2022, 12, 2.
- Puzzo, D.; Privitera, L.; Leznik, E.; Fà, M.; Staniszewski, A.; Palmeri, A.; Arancio, O. Picomolar Amyloid-β Positively Modulates Synaptic Plasticity and Memory in Hippocampus. J. Neurosci. 2008, 28, 14537–14545.
- Puzzo, D.; Privitera, L.; Fa’, M.; Staniszewski, A.; Hashimoto, G.; Aziz, F.; Sakurai, M.; Ribe, E.M.; Troy, C.M.; Mercken, M.; et al. Endogenous amyloid-β is necessary for hippocampal synaptic plasticity and memory. Ann. Neurol. 2011, 69, 819–830.
- Puzzo, D.; Arancio, O. Amyloid-β Peptide: Dr. Jekyll or Mr. Hyde? J. Alzheimer’s Dis. 2013, 33, S111–S120.
- Iqbal, K.; Liu, F.; Gong, C.-X. Tau and neurodegenerative disease: The story so far. Nat. Rev. Neurol. 2016, 12, 15–27.
- E Golde, T. Open questions for Alzheimer’s disease immunotherapy. Alzheimer’s Res. Ther. 2014, 6, 3.
- Rankovic, Z. CNS Drug Design: Balancing Physicochemical Properties for Optimal Brain Exposure. J. Med. Chem. 2015, 58, 2584–2608.
- Hubbard, R.E. Structure-based drug discovery and protein targets in the CNS. Neuropharmacology 2011, 60, 7–23.
- Liu, X.; Naomi, S.S.M.; Sharon, W.L.; Russell, E.J. The Applications of Focused Ultrasound (FUS) in Alzheimer’s Disease Treatment: A Systematic Review on Both Animal and Human Studies. Aging Dis. 2021, 12, 1977.
- Ringman, J.M.; Network, D.I.A.; Goate, A.; Masters, C.L.; Cairns, N.J.; Danek, A.; Graff-Radford, N.; Ghetti, B.; Morris, J.C. Genetic Heterogeneity in Alzheimer Disease and Implications for Treatment Strategies. Curr. Neurol. Neurosci. Rep. 2014, 14, 499.
- Schneider, L.S.; Mangialasche, F.; Andreasen, N.; Feldman, H.; Giacobini, E.; Jones, R.; Mantua, V.; Mecocci, P.; Pani, L.; Winblad, B.; et al. Clinical trials and late-stage drug development for Alzheimer’s disease: An appraisal from 1984 to 2014. J. Intern. Med. 2014, 275, 251–283.
- Duara, R.; Barker, W. Heterogeneity in Alzheimer’s Disease Diagnosis and Progression Rates: Implications for Therapeutic Trials. Neurotherapeutics 2022, 1–18.
- Giacobini, E.; Gold, G. Alzheimer disease therapy—Moving from amyloid-β to tau. Nat. Rev. Neurol. 2013, 9, 677–686.
- Bateman, R.J.; Xiong, C.; Benzinger, T.L.S.; Fagan, A.M.; Goate, A.; Fox, N.C.; Marcus, D.S.; Cairns, N.J.; Xie, X.; Blazey, T.M.; et al. Clinical and Biomarker Changes in Dominantly Inherited Alzheimer’s Disease. N. Engl. J. Med. 2012, 367, 795–804.
- Zetterberg, H.; Blennow, K.; Rinne, J.O.; Salloway, S.; Wei, J.; Black, R.; Grundman, M.; Liu, E.; for the AAB-001 201/202 Investigators. Effect of Immunotherapy with Bapineuzumab on Cerebrospinal Fluid Biomarker Levels in Patients With Mild to Moderate Alzheimer Disease. Arch. Neurol. 2012, 69, 1002–1010.
- Maurer, S.V.; Williams, C.L. The Cholinergic System Modulates Memory and Hippocampal Plasticity via Its Interactions with Non-Neuronal Cells. Front. Immunol. 2017, 8, 1489.
- Giacobini, E. Cholinesterase inhibitors: New roles and therapeutic alternatives. Pharmacol. Res. 2004, 50, 433–440.
- Folch, J.; Petrov, D.; Ettcheto, M.; Abad, S.; López, E.S.; García, M.L.; Olloquequi, J.; Beas-Zarate, C.; Auladell, C.; Camins, A. Current Research Therapeutic Strategies for Alzheimer’s Disease Treatment. Neural Plast. 2016, 2016, 8501693.
- Proskurnina, N.; Areshknina, L. Alkaloids of Galanthus woronovi. Allg. Chem. 1947, 17, 1216–1219.
- Mucke, H.A. The case of galantamine: Repurposing and late blooming of a cholinergic drug. Future Sci. OA 2015, 1, FSO73.
- Proskurnina, N.; Yakovieva, A. Alkaloids of Galanthus woronovi. Structure of galanthine. Ber. Akad. Wiss. UdSSR 1953, 90, 565–567.
- Uyeo, S.; Kobayashi, S. Lycoris Alkaloids. XXIV.: Isolation and Characterization of Lycoremine. Pharm. Bull. 1953, 1, 139–142.
- Irwin, R.; Smith, H. Cholinesterase inhibition by galanthamine and lycoramine. Biochem. Pharmacol. 1960, 3, 147–148.
- Lombardo, G.; Arena, G. The use of galantamine bromhydrate (nivaline) in the paralytic sequelae of poliomyelitis, neuraxitis and in muscular dystrophy. Minerva Pediatr. 1962, 30, 724–728.
- Stoyanov, E.; Vulchanova, S. The clinical application of nivalin as an antidote of curare. Nauchni Tr. Viss. Meditsinski Inst. Sofiia 1963, 42, 45–48.
- Baraka, A. Reversal of Central Anticholinergic Syndrome by Galanthamine. JAMA 1977, 238, 2293–2294.
- Czollner, L.; Frantsits, W.; Küenburg, B.; Hedenig, U.; Fröhlich, J.; Jordis, U. New kilogram-synthesis of the anti-alzheimer drug (−)-galanthamine. Tetrahedron Lett. 1998, 39, 2087–2088.
- Albuquerque, E.X.; Santos, M.D.; Alkondon, M.; Pereira, E.F.R.; Maelicke, A. Modulation of Nicotinic Receptor Activity in the Central Nervous System: A Novel Approach to the Treatment of Alzheimer Disease. Alzheimer Dis. Assoc. Disord. 2001, 15, S19–S25.
- Dajas-Bailador, F.A.; Heimala, K.; Wonnacott, S. The Allosteric Potentiation of Nicotinic Acetylcholine Receptors by Galantamine Is Transduced into Cellular Responses in Neurons: Ca2+ Signals and Neurotransmitter Release. Mol. Pharmacol. 2003, 64, 1217–1226.
- Samochocki, M.; Höffle, A.; Fehrenbacher, A.; Jostock, R.; Ludwig, J.; Christner, C.; Radina, M.; Zerlin, M.; Ullmer, C.; Pereira, E.F.R.; et al. Galantamine Is an Allosterically Potentiating Ligand of Neuronal Nicotinic but Not of Muscarinic Acetylcholine Receptors. J. Pharmacol. Exp. Ther. 2003, 305, 1024–1036.
- Schilström, B.; Ivanov, V.B.; Wiker, C.; Svensson, T.H. Galantamine Enhances Dopaminergic Neurotransmission In Vivo Via Allosteric Potentiation of Nicotinic Acetylcholine Receptors. Neuropsychopharmacology 2007, 32, 43–53.
- Lilienfeld, S. Galantamine—A Novel Cholinergic Drug with a Unique Dual Mode of Action for the Treatment of Patients with Alzheimer’s Disease. CNS Drug Rev. 2002, 8, 159–176.
- Tang, X.C.; Han, Y.F. Pharmacological Profile of Huperzine A, a Novel Acetylcholinesterase Inhibitor from Chinese Herb. CNS Drug Rev. 1999, 5, 281–300.
- Wang, Y.; Yue, D.; Tang, X. Anti-Cholinesterase Activity of Huerpzine A. Acta Pharmacol. Sin. 1986, 7, 110–113.
- Wang, H.; Tang, X. Anticholinesterase Effects of Huperzine A, E2020, and Tacrine in Rats. Zhongguo Yao Li Xue Bao Acta Pharmacol. Sin. 1998, 19, 27–30.
- Wang, R.; Tang, X.C. Neuroprotective Effects of Huperzine A. Neurosignals 2005, 14, 71–82.
- Zhang, H.Y.; Liang, Y.Q.; Tang, X.C.; He, X.C.; Bai, D.L. Stereoselectivities of enantiomers of huperzine A in protection against β-amyloid25–35-induced injury in PC12 and NG108-15 cells and cholinesterase inhibition in mice. Neurosci. Lett. 2002, 317, 143–146.
- Orhan, G.; Orhan, I.; Subutay-Oztekin, N.; Ak, F.; Sener, B. Contemporary Anticholinesterase Pharmaceuticals of Natural Origin and Their Synthetic Analogues for the Treatment of Alzheimers Disease. Recent Patents CNS Drug Discov. 2009, 4, 43–51.
- Zhan, Z.-J.; Bian, H.-L.; Wang, J.-W.; Shan, W.-G. Synthesis of physostigmine analogues and evaluation of their anticholinesterase activities. Bioorganic Med. Chem. Lett. 2010, 20, 1532–1534.
- McKinney, M.; Miller, J.H.; Yamada, F.; Tuckmantel, W.; Kozikowski, A.P. Potencies and stereoselectivities of enantiomers of huperzine A for inhibition of rat cortical acetylcholinesterase. Eur. J. Pharmacol. 1991, 203, 303–305.
- Dvir, H.; Jiang, H.L.; Wong, D.M.; Harel, M.; Chetrit, M.; He, X.C.; Jin, G.Y.; Yu, G.L.; Tang, X.C.; Silman, I.; et al. X-ray Structures of Torpedo californica Acetylcholinesterase Complexed with (+)-Huperzine A and (−)-Huperzine B: Structural Evidence for an Active Site Rearrangement. Biochemistry 2002, 41, 10810–10818.
- Zhang, H.-Y.; Brimijoin, S.; Tang, X.-C. Apoptosis induced by beta-amyloid25-35 in acetylcholinesterase-overexpressing neuroblastoma cells. Acta Pharmacol. Sin. 2003, 24, 853–858.
- Yan, X.F.; Lu, W.H.; Lou, W.J.; Tang, X.C. Effects of Huperzine A and B on Skeletal Muscle and the Electroenphalogram. Acta Pharmacol. Sin. 1987, 8, 117–123.
- Zhang, R.W.; Tang, X.C.; Han, Y.Y.; Sang, G.W.; Zhang, Y.D.; Ma, Y.X.; Zhang, C.L.; Yang, R.M. Drug evaluation of huperzine A in the treatment of senile memory disorders. Acta Pharmacol. Sin. 1991, 12, 250–252.
- Xu, S.S.; Gao, Z.X.; Weng, Z.; Du, Z.M.; Xu, W.A.; Yang, J.S.; Zhang, M.L.; Tong, Z.H.; Fang, Y.S.; Chai, X.S. Efficacy of Tablet Huperzine A on Memory, Cognition and Behavior in Alzheimer´s Disease. Acta Pharmacol. Sin. 1995, 16, 195–391.
- Xu, S.S.; Cai, Z.Y.; Qu, Z.W.; Yang, R.M.; Cai, Y.L.; Wang, G.Q.; Su, X.Q.; Zhong, X.S.; Cheng, R.Y.; A Xu, W.; et al. Huperzine-A in capsules and tablets for treating patients with Alzheimer disease. Acta Pharmacol. Sin. 1999, 20, 486–490.
- Liao, B.; Newmark, H.; Zhou, R. Neuroprotective Effects of Ginseng Total Saponin and Ginsenosides Rb1 and Rg1 on Spinal Cord Neurons In Vitro. Exp. Neurol. 2002, 173, 224–234.
- Gillis, C. Panax ginseng pharmacology: A nitric oxide link? Biochem. Pharmacol. 1997, 54, 1–8.
- Pena, I.D.; Yoon, S.Y.; Kim, H.J.; Park, S.; Hong, E.Y.; Ryu, J.H.; Park, I.H.; Cheong, J.H. Effects of ginseol k-g3, an Rg3-enriched fraction, on scopolamine-induced memory impairment and learning deficit in mice. J. Ginseng Res. 2014, 38, 1–7.
- Rausch, W.-D.; Liu, S.; Gille, G.; Radad, K. Neuroprotective effects of ginsenosides. Acta Neurobiol. Exp. 2006, 66, 369–375.
- Nah, S.-Y.; Kim, D.-H.; Rhim, H. Ginsenosides: Are Any of them Candidates for Drugs Acting on the Central Nervous System? CNS Drug Rev. 2007, 13, 381–404.
- Radad, K.; Moldzio, R.; Rausch, W.-D. Ginsenosides and Their CNS Targets. CNS Neurosci. Ther. 2011, 17, 761–768.
- Lee, D.W.; Andersen, J.K. Iron elevations in the aging Parkinsonian brain: A consequence of impaired iron homeostasis? J. Neurochem. 2010, 112, 332–339.
- Selkoe, D.J.; Schenk, D. Alzheimer’s Disease: Molecular Understanding Predicts Amyloid-Based Therapeutics. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 545–584.
- Dedov, V.N.; Griffiths, F.M.; Garner, B.; Halliday, G.M.; Double, K.L. Lipid content determines aggregation of neuromelanin granules in vitro. J. Neural. Transm. Suppl. 2007, 72, 35–38.
- Tanzi, R.E.; Bertram, L. Alzheimer´s Disease: The latest suspect. Nature 2008, 454, 707–708.
- Xu, B.-B.; Liu, C.-Q.; Gao, X.; Zhang, W.-Q.; Wang, S.-W.; Cao, Y.-L. Possible mechanisms of the protection of ginsenoside Re against MPTP-induced apoptosis in substantia nigra neurons of Parkinson’s disease mouse model. J. Asian Nat. Prod. Res. 2005, 7, 215–224.
- Oh, J.; Kim, J.-S. Compound K derived from ginseng: Neuroprotection and cognitive improvement. Food Funct. 2016, 7, 4506–4515.
- Fang, F.; Chen, X.; Huang, T.; Lue, L.-F.; Luddy, J.S.; Yan, S.S. Multi-faced neuroprotective effects of Ginsenoside Rg1 in an Alzheimer mouse model. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2012, 1822, 286–292.
- Hong-Cai, W.; Yu-Meng, J.; Xue, Z.; Ning, W.; Di, G.; Ming, G.; Jing-Yu, Z.; Zi-Chao, Y. Protective Effects of Ginsenoside Rb1 on Aβ Amyloid-Induced Hippocampal Neuronal Injury in Rats. J. Jilin Univ. Med. Ed. 2012, 38, 447–450.
- Huang, X.; Li, N.; Pu, Y.; Zhang, T.; Wang, B. Neuroprotective Effects of Ginseng Phytochemicals: Recent Perspectives. Molecules 2019, 24, 2939.
- Zhao, H.-H.; Di, J.; Liu, W.-S.; Liu, H.-L.; Lai, H.; Lü, Y.-L. Involvement of GSK3 and PP2A in ginsenoside Rb1’s attenuation of aluminum-induced tau hyperphosphorylation. Behav. Brain Res. 2013, 241, 228–234.
- Oken, B.S.; Storzbach, D.M.; Kaye, J.A. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch. Neurol. 1998, 55, 1409–1415.
- Vellas, B.; Coley, N.; Ousset, P.-J.; Berrut, G.; Dartigues, J.-F.; Dubois, B.; Grandjean, H.; Pasquier, F.; Piette, F.; Robert, P.; et al. Long-term use of standardised ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): A randomised placebo-controlled trial. Lancet Neurol. 2012, 11, 851–859.
- Singh, S.K.; Srivastav, S.; Castellani, R.J.; Plascencia-Villa, G.; Perry, G. Neuroprotective and Antioxidant Effect of Ginkgo biloba Extract Against AD and Other Neurological Disorders. Neurotherapeutics 2019, 16, 666–674.
- Smith, P.F.; Maclennan, K.; Darlington, C.L. The neuroprotective properties of the Ginkgo biloba leaf: A review of the possible relationship to platelet-activating factor (PAF). J. Ethnopharmacol. 1996, 50, 131–139.
- Shi, C.; Liu, J.; Wu, F.; Yew, D.T. Ginkgo biloba Extract in Alzheimer’s Disease: From Action Mechanisms to Medical Practice. Int. J. Mol. Sci. 2010, 11, 107–123.
- Augustin, S.; Rimbach, G.; Augustin, K.; Schliebs, R.; Wolffram, S.; Cermak, R. Effect of a short- and long-term treatment with Ginkgo biloba extract on Amyloid Precursor Protein Levels in a transgenic mouse model relevant to Alzheimer’s disease. Arch. Biochem. Biophys. 2009, 481, 177–182.
- Löffler, T.; Lee, S.K.; Nöldner, M.; Chatterjee, S.S.; Hoyer, S.; Schliebs, R. Effect of Ginkgo biloba extract (EGb761) on glucose metabolism-related markers in streptozotocin-damaged rat brain. J. Neural Transm. 2001, 108, 1457–1474.
- Le Bars, P.L.; Katz, M.M.; Berman, N.; Itil, T.M.; Freedman, A.M.; Schatzberg, A.F. A Placebo-Controlled, Double-blind, Randomized Trial of an Extract of Ginkgo Biloba for Dementia. JAMA 1997, 278, 1327–1332.
- Mix, J.A.; Crews, W.D. A double-blind, placebo-controlled, randomized trial of Ginkgo biloba extract EGb 761® in a sample of cognitively intact older adults: Neuropsychological findings. Hum. Psychopharmacol. Clin. Exp. 2002, 17, 267–277.
- Kanowski, S.; Herrmann, W.M.; Stephan, K.; Wierich, W.; Hörr, R. Proof of Efficacy of the Ginkgo Biloba Special Extract EGb 761 in Outpatients Suffering from Mild to Moderate Primary Degenerative Dementia of the Alzheimer Type or Multi-infarct Dementia. Pharmacopsychiatry 2007, 29, 47–56.
- Napryeyenko, O.; Borzenko, I. Ginkgo biloba Special Extract in Dementia with Neuropsychiatric Features. Arzneimittelforschung 2007, 57, 4–11.
- Gao, Y.; Zhang, L.; Jiao, W. Marine glycan-derived therapeutics in China. Prog. Mol. Biol. Transl. Sci. 2019, 163, 113–134.
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019, 29, 787–803.
- Syed, Y.Y. Sodium Oligomannate: First Approval. Drugs 2020, 80, 441–444.
- Xiao, S.; Chan, P.; Wang, T.; Hong, Z.; Wang, S.; Kuang, W.; He, J.; Pan, X.; Zhou, Y.; Ji, Y.; et al. A 36-week multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 3 clinical trial of sodium oligomannate for mild-to-moderate Alzheimer’s dementia. Alzheimer’s Res. Ther. 2021, 13, 62.
- Isaka, S.; Cho, K.; Nakazono, S.; Abu, R.; Ueno, M.; Kim, D.; Oda, T. Antioxidant and anti-inflammatory activities of porphyran isolated from discolored nori (Porphyra yezoensis). Int. J. Biol. Macromol. 2015, 74, 68–75.
- Kim, M.; Li, Y.-X.; Dewapriya, P.; Ryu, B.; Kim, S.-K. Floridoside suppresses pro-inflammatory responses by blocking MAPK signaling in activated microglia. BMB Rep. 2013, 46, 398–403.
- Cui, Y.-Q.; Jia, Y.-J.; Zhang, T.; Zhang, Q.-B.; Wang, X.-M. Fucoidan Protects against Lipopolysaccharide-Induced Rat Neuronal Damage and Inhibits the Production of Proinflammatory Mediators in Primary Microglia. CNS Neurosci. Ther. 2012, 18, 827–833.
- Park, H.Y.; Han, M.H.; Park, C.; Jin, C.-Y.; Kim, G.-Y.; Choi, I.-W.; Kim, N.D.; Nam, T.-J.; Kwon, T.K.; Choi, Y.H. Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem. Toxicol. 2011, 49, 1745–1752.
- Yao, Z.-A.; Xu, L.; Wu, H.-G. Immunomodulatory Function of κ-Carrageenan Oligosaccharides Acting on LPS-Activated Microglial Cells. Neurochem. Res. 2014, 39, 333–343.