Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Ovarian cancer is a lethal gynecologic tumor and is generally resistant to conventional treatments. Stable cancer-associated fibroblasts (CAFs) are important cellular components in the ovarian cancer tumor microenvironment and may provide novel resources for future treatment strategies. Different subtypes of CAFs display specific functions in tumor pathogenesis and various CAF markers suggest potential treatment targets. Several clinical or preclinical trials have targeted stromal fibroblasts and focused on the properties of CAFs to enhance ovarian cancer treatment efficacy.
- cancer-associated fibroblasts
- ovarian cancer
- tumor microenvironment
- stroma
1. Introduction
Globally, there are about 300,000 new cases of and 185,000 deaths due to ovarian cancer, making it the third most common gynecologic cancer and the second leading cause of reproductive cancer death among women [1][2]. In 2015, there were about 52,100 new cases of and 22,500 deaths caused by ovarian cancer in China [3], causing significant health issues and seriously threatening women’s health. Due to various pathological types, limited screening methods, and lack of distinct clinical manifestations, 75% of patients are diagnosed at an advanced stage and the prognosis is not optimistic [4]. At present, the mainstay treatment of ovarian cancer is surgical cytoreduction to R0, followed by carboplatin and paclitaxel combined chemotherapy [5]. Further maintenance therapies may be applied after first-line chemotherapy, which include poly ADP-ribose polymerase (PARP) inhibitors, anti-angiogenesis agents or monoclonal antibodies [6]. Although more than 80% of patients are initially sensitive to treatment, the majority of them go on to develop chemotherapy resistance, resulting in advanced recurrence and eventually death [7]. It is reported that the overall 5-year survival rate of advanced patients is approximately 30%, and prognoses are not optimistic even in high-resource countries such as the United States, where the rate is only about 47% [1]. Therefore, there is an urgent need to better understand the heterogeneity and biology of ovarian cancer so as to develop or refine treatment strategies and improve quality of life.
Since Paget first proposed the ‘Seed and Soil’ theory in 1889 [8], it has been gradually recognized that apart from the cancer cells, stable stromal cells with few mutations in the tumor microenvironment (TME) are also of great importance [9]. The interplay between tumor and stromal cells contributes greatly to tumorigenesis, progression, and therapeutic resistance [10]. Some of the most significant stromal components are cancer-associated fibroblasts (CAFs), which modulate cancer progression and treatment responses in many cancer types [10][11]. CAFs can form robust crosstalk with cancer cells, participate in various biological processes, including wound healing and inflammatory processes, tumor initiation, progression, and immune exclusion, and may also contribute to therapeutic failure, especially chemoresistance [10][11][12]. Therefore, CAFs are considered to be notable tumor-promoting players. Treatment barriers in ovarian cancer have been increasingly ascribed to angiogenesis, stromal reprogramming, extracellular matrix remodeling (ECM), and drug delivery [13][14]. In principle, a better understanding of the intricate mechanisms of CAFs in tumor pathophysiology could pave the way for CAF-targeted therapeutics. Interestingly, several studies focusing on Shh-deficient signaling indicated that, besides supporting tumor pathogenesis, CAFs also had tumor-restraining functions [15][16]. A population-based study has demonstrated improved 10-year survival in breast cancer patients receiving breast-conserving surgery plus radiotherapy compared with mastectomy, which may partially be explained by remnant tumor stroma after surgery. The stromal components could induce long-lasting antitumor immunity and restrain tumor progression, and therefore may improve clinical outcomes [17][18]. The available evidence has suggested that in-depth studies of CAFs may aid in elucidating the mechanisms of ovarian cancer progression, dissemination, and therapeutic resistance.
2. The Role of CAFs in the Therapeutic Resistance of Ovarian Cancer
Chemotherapy plays an important role in the comprehensive treatment of ovarian cancer. Although more than 80% of patients respond well to treatment at an early stage, most patients develop drug resistance at the late stage and eventually suffer relapse and/or death [7]. Although mechanisms of drug resistance are still elusive, immune exclusion triggered by Treg or other immune components may play an important role [19][20]. The formation of extracellular matrices can interfere with drug transport and affect drug efficacy [20][21]. Some specific CAFs subtypes, such as CD10+ GPR77+ CAFs, or CAFs markers, such as CD44, may promote chemoresistance by sustaining cancer cell stemness [22][23]. Other mechanisms may be related to metabolic activities, such as autophagy and inhibition of cancer cell apoptosis [24][25][26]. Single-cell RNA sequencing (scRNA-seq) has been applied to analyze fibroblast heterogeneity. Results have shown that CAF-S1, which is characterized by TGF-β signaling, is correlated with immunosuppression and indicates primary resistance to chemotherapy. CAF-S1 upregulates the protein levels of PD-1 and CTLA-4 in regulatory T lymphocytes (Treg), which, in turn, increases levels of CAF-S1. On the other hand, upregulated levels of PD-1 and CTLA-4 proteins may serve as promising checkpoints which may pave the way for combining immunotherapy with targeted therapies [19]. Many chemotherapeutic agents must pass through the blood vessels and ECM to reach a tumor and exert effects. During this process, CAFs can obstruct the transportation of chemotherapeutic agents and impair chemotherapeutic efficacy by creating physical barriers and microvascular compression. For example, cysteine and glutathione produced by CAFs can inhibit the accumulation of cisplatin in the nucleus of ovarian cancer, resulting in platinum resistance [20]. Effector CD8+ T lymphocytes could affect the metabolic process of cysteine and glutathione in fibroblasts, creating chemoresistance via the JAK/STAT1 pathway or lowering medication efficacy by modifying cysteine and glutathione metabolism [20]. NF-κB continuously activates CD10+ GPR77+ CAFs through p56 phosphorylation and acetylation. Maintained by complementary Ca5 and GPR77 receptor signaling, CD10+ GPR77+ CAF activation provides a supportive environment for tumor stem cells and chemoresistance [22]. It has also been observed that CAFs derived from wild-type mice express high levels of CD44 in hypoxic and low-nutrition environments, which could maintain the stemness of cancer stem cells, while CD44-deficient CAFs do not have these properties [23]. Another study of CAFs and SKOV3 ovarian tumor cells has shown that increased level of ROS could promote autophagy of tumor cells and enhance the expression of drug resistance-related genes, such as YAP, CTGF, and Cyr61 [24]. Functional studies have also confirmed that microRNA-21 (miR21) in exosomes transferred from neighboring CAFs could inhibit ovarian cancer apoptosis and generate chemotherapy failure [25], which has provided an alternative strategy for suppressing tumorigenesis and treatment resistance. In addition, CAFs could also affect ovarian cancer chemotherapy resistance by directly acting on XIAP and regulating the PI3K/AKT signaling pathway [26]. Many chemotherapeutic agents must be degraded into active forms to exert their antitumor effects. For example, gemcitabine, a pyrimidine antagonist, should be metabolized intracellularly to active metabolites to inhibit DNA synthesis. Gemcitabine is used in the treatment of ovarian cancer, as a single agent or together with carboplatin and/or bevacizumab in recurrent ovarian cancer, with survival benefits reported in clinical trials [27][28]. However, studies directly investigating the effects of gemcitabine on CAFs have mostly examined pancreatic cancers. TGF-β-induced stromal CYR61 negatively regulates the expression of the nucleoside transporters Hent1 and Hcnt3 in pancreatic tumor cells and significantly reduces the uptake of gemcitabine by cells, which is linked to gemcitabine resistance [21]. Although radiotherapy is not a standard treatment for ovarian cancer, it is also used when single recurrences occur after first-line treatment [29]. Similar to chemotherapy, CAFs have been proven to regulate the radiation response of malignant tumors via a paracrine mode or direct interactions during tumor radiotherapy [30]. Chemotherapy, radiotherapy, and immunotherapy all induce DNA damage in tumor cells, so survival and apoptosis-related signals can also affect the resistance to radiation-induced DNA damage, such as CAFs stimulation of MAPK, the AKT signaling pathway, and the β1-integrin-FAK signaling pathway [31]. It has been reported that TGF-β accelerates EMT progression and causes E-cadherin loss, and therefore induces radiotherapy tolerance in pancreatic cancer [32][33]. Additionally, CAFs affect the reactivity of tumor cells to checkpoint inhibitors by forming immunosuppressive tumor microenvironments. For example, in pancreatic ductal carcinoma, CXCL12 from FAP+ CAFs can wrap tumor cells and inhibit the accumulation of T cells in tumor sites. Depletion of FAP+ CAFs enables immunological checkpoint antagonists, such as α-CTLA-4 and α-PD-L1, to exert antitumor effects. CXCL-12 receptor 4 (CXCR4) inhibitors can be applied to recruit T cells to accumulate in cancer cells as well as cooperate with α-PD-L1 to significantly reduce the number of tumor cells [34]. What is more, high-dose radiotherapy can reprogram cancer immunity to activate both innate and adaptive immunity by upregulating the expression of MHC class I molecules and death receptors on tumor cells [35].
3. Therapeutic Prospective of CAFs in Ovarian Cancer
With an increased understanding of CAFs, it is believed that CAFs may serve as promising targets for ovarian cancer therapeutics. Many studies in this entry have suggested potential targets as treatment strategies for ovarian cancer, one of which, involving the targeting of TGF-β 1 and 2, is currently under investigation in clinical trials (Phase 2 Trial of Maintenance Vigil for High-Risk Stage IIIb–IV Ovarian Cancer (VITAL), NCT02346747) [36] (Figure 1). In addition, the most common inhibitors targeting CAFs are summarized in Table 1.
CAFs: cancer-associated fibroblasts.
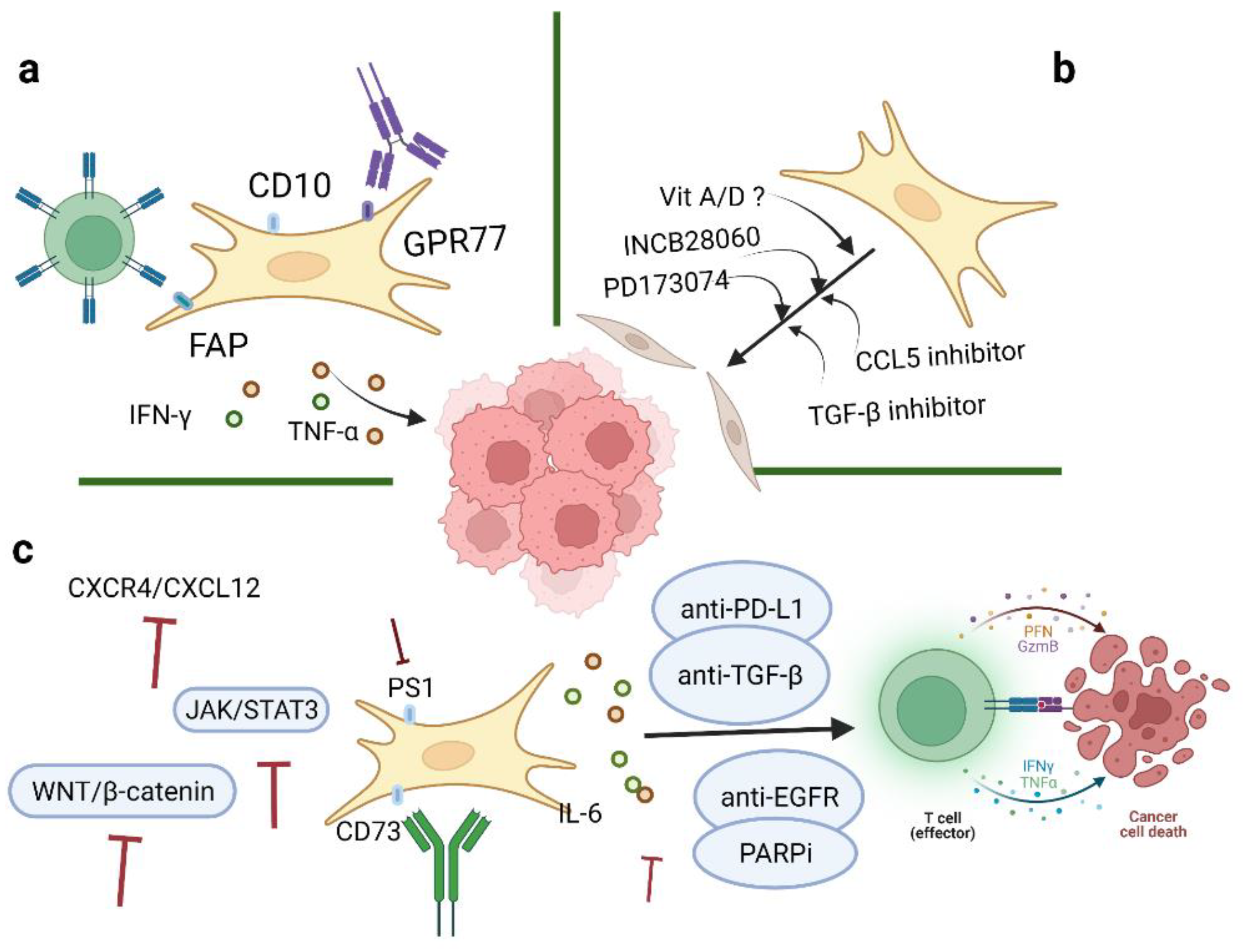
Figure 1. Therapeutic strategies of CAFs in ovarian cancer. Three aspects of strategies targeting CAFs in TME include: (a) Depletion of CAFs via specific surface markers; (b) conversion of activated CAFs to the quiescent state by inhibitors; and (c) targeting significant downstream effectors of CAFs. Image created with BioRender.com (accessed on 23 May 2022). CAFs: cancer-associated fibroblasts; TME: tumor microenvironment.
Table 1. CAFs-associated inhibitors in ovarian cancer.
| Inhibitors | Targets | Functions | Reference |
|---|---|---|---|
| Tocilizumab | IL-6R | Promotes anti-tumor immunity | [37] |
| INCB28060 | HGF and c-Met | Blocks chemotherapeutic failure | [38] |
| PD173074 | FGF | Terminates cellular proliferation and migration | [39] |
| Galunisertib | TGF-β1 | Reverses MHC-I loss caused by TGF-β | [40] |
| DNMT | MHC-I | Inhibits tumor-immune excluded subtype | [41] |
| MPDL3280A | PD-L1 | Suppresses T cell migration, proliferation, and secretion of cytotoxic mediators and restricts tumor cell killing | [42] |
| Vigil | TGFβ-1 and TGFβ-2 | Downregulates TGF-β 1 and 2; provides personal neoantigen; induces GMCSF expression |
[43] |
| AG1478 | EGFR | Upregulates autophagy levels | [44] |
| Elaiophylin | Autophagy | Inhibits activation of autophagy | [45] |
| Bevacizumab | VEGF | Inhibits tumor angiogenesis | [27][46][47] |
CAFs: cancer-associated fibroblasts.
3.1. Depletion of CAFs via Specific Surface Markers
Current evidence has indicated that CAFs are important mediators of proliferation, migration, and invasiveness in ovarian cancer. Four CAFs phenotypes in ovarian cancer have been characterized, mainly by the expression levels of α-SMA, FAP, and CD29. Direct CAF-depletion treatment strategies mainly target CAF surface markers, such as α-SMA, FAP, and GPR77 (Figure 1a). In a transgenic mouse model of PDAC, selective depletion of α-SMA could not only restrain angiogenesis but also enhance hypoxia and induce epithelial-to-mesenchymal transition (EMT) and stemness, thus leading to reduced survival [15]. Moreover, knockout of CAFs alters CD4+Foxp3+ Treg composition to inhibit immune surveillance, thus impeding the response of myofibroblast-depleted tumors to gemcitabine [15]. Despite this, anti-CTLA-4 immunotherapy effectively abrogates tumor development and prolongs survival in mice [15]. However, the clinical outcomes of targeting α-SMA seem to be unsatisfactory; another CAF treatment strategy is to target FAP. Depletion of FAP-expressing CAFs can cause hypoxic necrosis of cancer and stromal cells via interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) [48]. At the same time, FAP-silenced SKOV3 cells can reduce tumor growth and inhibit CAF infiltration [49]. However, FAP is not specifically expressed by CAFs, which may affect the accuracy of CAF-targeted therapies. One study has shown that CD10+ GPR77+ CAFs can promote successful implantation in xenotransplantation and that targeting GPR77 could inhibit tumor formation and restore tumor chemosensitivity [22]. On the basis of these findings, the researchers hypothesize that depletion of CD10 and/or GPR77 may suppress the tumor-promoting roles of CAFs in ovarian cancer. Further studies are needed to better elucidate the mechanism and function of other specific markers of CAFs. Another strategy to reduce CAFs in tumors is to target potential cellular sources of CAFs. For example, endothelial cells are a potential source of CAFs in malignant pleural mesothelioma (MPeM), and VEGF is a key mitogen in MPeM [46]. Bevacizumab is an antiangiogenic agent against VEGF that can inhibit angiogenesis and tumor growth to reduce CAF infiltration in tumor sites. A phase III clinical study targeting CAF precursors with bevacizumab in MPeM has reported improved overall survival but also observed toxic effects [46]. Bevacizumab is the only approved antiangiogenic agent in ovarian cancer treatment. Several clinical trials have reported that the application of bevacizumab after chemotherapy (carboplatin and paclitaxel) has improved progression-free survival in ovarian cancer [27][47]. CAFs can also secrete small extracellular vesicles (sEVs) which could transport growth factors to target cells. In oral squamous cell carcinoma (SOCC), VEGF released from CAFs could bind to sEVs and stimulate angiogenesis and impair cell sensitivity to bevacizumab [50]. Furthermore, heparinase could release VEGF from sEVs and bevacizumab could neutralize the dissociated VEGF and inhibit angiogenesis [50].
3.2. Conversion of Activated CAFs to the Quiescent State
Besides direct depletion of CAFs, another therapeutic strategy is to revert activated CAFs to the quiescent state or induce their functioning as tumor-suppressive players (Figure 1b). A number of cytokines or pathways are involved in the CAFs activation process. For example, microRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer, especially by downregulation of miR-31 and miR-214 and upregulation of miR-155. CCL5, a direct candidate target of miR214, can contribute to suppressing tumor growth and metastasis [51]. Ovarian cancer-related fibroblasts have been shown to exhibit resistance to PARP inhibitors (PARPis). At the same time, the activation of PARPi-induced stromal fibroblasts requires increased CCL5 secretion by the activation of NF-κB signaling [52]. Therefore, neutralizing CCL5 may facilitate the transition of CAFs from an activated state to a quiescent state. In addition, TGF-β may contribute to the activation of CAFs and the progression of ovarian cancer. Two autocrine loops, mediated by TGF-β and SDF-1, are stimulated to initiate and maintain the differentiation of human mammary fibroblasts into activated CAFs, which have tumor-promoting features [53]. Targeting TGF-β could weaken both the expression of CXCR4 and activation of TβR–Smad signaling in stromal fibroblasts, thus hindering the feedback loop of TGF-β and SDF-1 and blocking the activation of tumor-promoting CAFs [53]. The targeting of other related genes, such as MYC and VCAN, may serve as potential therapeutic targets for ovarian cancer [54][55]. Indeed, treatment with vitamin D in PDAC has shown a 57% increase in survival compared to chemotherapy alone in mice [56]. Application of vitamin A has also been demonstrated to improve survival in PDAC [57]. Therefore, the researchers hypothesize that vitamins can be used as adjuncts to current ovarian cancer treatments to enhance their therapeutic efficacy.
3.3. Targeting Significant Downstream Effectors of CAFs
Challenges remain in the development of strategies for CAF depletion or differentiation into a quiescent state. Therefore, alternative strategies have been developed to target other important effectors involved in CAFs biological processes (Figure 1c). Studies have shown that TGF-β plays multifaceted roles in defining tumor-immune phenotypes. CAFs are more sensitive to immune checkpoint inhibitors (ICIs) in immune-infiltrated tumors due to higher antigen presentation, PD-L1 expression, and immune features [58]. TGF-β is correlated with weak responses to ICI therapy in immune-desert and immune-excluded tumors [59]. Coadministration of TGF-β inhibitors and anti-PD-L1 antibodies can exhibit anti-tumor immunity by facilitating T cell infiltration in tumor sites [42]. TGF-β is highly expressed in ovarian cancer and is essential for activating CAFs, facilitating tumor pathogenesis processes, averting immune regulation, and eventually forming a favorable TME. A clinical trial of an autologous tumor vaccine named gemogenovatucel-T (Vigil) (NCT02346747) has shown that Vigil provided personal neoantigens, downregulated TGF-β 1 and 2 and induced GMCSF expression, leading to systemic immunosuppressive activities. Current results have indicated long-term beneficial effects of Vigil with respect to primary endpoints and support further investigation of Vigil in ovarian cancer [43]. Studies have found that inhibiting the CXCR4/CXCL12 axis can increase sensitivity to anti-PD-1 and anti-CTLA4 immunological therapies [34][60]. IL-6 is specially induced by IL-1β, TNF-α, and TGF-β signaling. Elevated IL-6 levels in serum and ascites have been shown to be associated with impaired cancer cell sensitivity to chemotherapy and poor prognosis in ovarian cancer patients. IL-6 can induce the overexpression of MMP, promote EMT, and activate the MAPK, PI3K/AKT, or JAK/STAT3 pathways to enhance tumor growth and cause chemoresistance [61]. Tocilizumab, an IL-6R antibody, could induce the production of tumor immunity-stimulating factors, such as IL-12, IL-1β, and anti-tumor cytokines, such as TNF-α, and IFN-γ. Clinical trial results have revealed that the combination of Tocilizumab with chemotherapy showed a good safety profile and possibly restored cancer cell sensitivities to chemotherapeutic agents and achieved immunological benefits [37][62]. However, there are certain limitations to these studies, such as small numbers of enrolled patients, serum levels detected at different FIGO stages, and no parallel analysis of IL-6 with other cytokines [62]. Therefore, further studies are needed to clarify the roles of IL-6 and IL-6R in ovarian cancer pathogenesis and treatment responses. It is reported that PS1 is highly expressed in CAFs and plays an important role in regulating effector CD8+ T cells in ovarian cancer. Notably, when PS-1 expression is silenced, immunosuppressive IL-1β is downregulated via the WNT/β-catenin pathway and consequently contributes to the proliferation of functional CTLs to improve the efficacy of immunotherapies [63]. Another important effector associated with CAF functions is the immunosuppressive enzyme CD73 on the surface of CAFs. In vitro, CD73 and extracellular adenosine can promote tumor growth and induce the expression of the BCL-2 anti-apoptotic family [64]. There are studies that have applied Hedgehog inhibitors in attempts to restrain tumor growth in gastrointestinal cancer. One study has shown that Hedgehog signaling inhibitors are associated with alterations of fibroblast composition and effector T cell infiltration and that the altered microenvironment facilitates pancreatic cancer progression [65][66]. Meanwhile, autophagy plays an important role in regulating the metabolic crosstalk between ovarian cancer cells and cancer-associated fibroblasts. In this respect, therapeutic options are referred to both induction and inhibition of autophagy. Compared to monotherapy, combined administration of PARPi and EGFR inhibitors has shown better antitumor effects in ovarian cancer A2780 xenografts, mainly through the activation of the ERK (MAPK) and JNK and inhibition of the AKT/mTOR/(p70S6K) pathways [44]. Interestingly, suppression of autophagy is partly associated with the improved efficacy of cisplatin in cisplatin-resistant ovarian cancer cells. For example, the autophagy inhibitor elaiophylin can exhibit anti-tumor activities as a single agent and can also decrease cell viability in combination with cisplatin. Therefore, elaiophylin has the potential to be a treatment agent for ovarian cancer [45].
Compared with monotherapy, combinations of chemotherapy, immunotherapy, and/or radiotherapy may achieve better therapeutic efficacy. Studies have shown that fibroblasts inhibit platinum aggregation in tumor nuclei by producing cysteine and glutathione, while CD8+ T cells can produce γ-interferon to upregulate γ-glutamyltransferase and inhibit the transcription of xc (−) cystine and glutamate receptors through the JAK/STAT1 pathway, thus controlling the synthesis of glutathione and cysteine in fibroblasts and effectively eliminating chemotherapy resistance in matrices [20]. Recent clinical trial results have reported that the application of bevacizumab after chemotherapy has been associated with survival benefits in ovarian cancer patients. Benefits have also been observed when bevacizumab has been used in combination with PARPis [27][47]. Antiangiogenics can enhance cancer immunotherapy by increasing immune cell infiltration and reducing immunosuppression in the TME [27]. In immune-desert tumors, low-dose radiotherapy combined with immunotherapy could reprogram the microenvironment and mobilize both innate and adaptive cells to achieve NKG2D-dependent anti-tumor immunity [29].
This entry is adapted from the peer-reviewed paper 10.3390/cancers14112637
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253.
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132.
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019, 30, 672–705.
- Piver, M.S. Treatment of ovarian cancer at the crossroads: 50 years after single-agent melphalan chemotherapy. Oncology 2006, 20, 1156–1158.
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Chen, L.M.; Cristea, M.; DeRosa, M.; Eisenhauer, E.L.; et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 191–226.
- Odunsi, K. Immunotherapy in ovarian cancer. Ann. Oncol. 2017, 28, viii1–viii7.
- Paget, S. The distribution of secondary growths in cancer of the breast. Lancet 1889, 133, 571–573.
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186.
- Polyak, K.; Haviv, I.; Campbell, I.G. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009, 25, 30–38.
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598.
- Biffi, G.; Tuveson, D.A. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol. Rev. 2021, 101, 147–176.
- Zanotelli, M.R.; Reinhart-King, C.A. Mechanical Forces in Tumor Angiogenesis. Adv. Exp. Med. Biol. 2018, 1092, 91–112.
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.C.; LeBleu, V.S.; Kalluri, R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530.
- Özdemir, B.C.; Pentcheva-Hoang, T.; Carstens, J.L.; Zheng, X.; Wu, C.C.; Simpson, T.R.; Laklai, H.; Sugimoto, H.; Kahlert, C.; Novitskiy, S.V.; et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014, 25, 719–734.
- Rhim, A.D.; Oberstein, P.E.; Thomas, D.H.; Mirek, E.T.; Palermo, C.F.; Sastra, S.A.; Dekleva, E.N.; Saunders, T.; Becerra, C.P.; Tattersall, I.W.; et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014, 25, 735–747.
- Strom, T.; Harrison, L.B.; Giuliano, A.R.; Schell, M.J.; Eschrich, S.A.; Berglund, A.; Fulp, W.; Thapa, R.; Coppola, D.; Kim, S.; et al. Tumour radiosensitivity is associated with immune activation in solid tumours. Eur. J. Cancer 2017, 84, 304–314.
- van Maaren, M.C.; de Munck, L.; de Bock, G.H.; Jobsen, J.J.; van Dalen, T.; Linn, S.C.; Poortmans, P.; Strobbe, L.J.A.; Siesling, S. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: A population-based study. Lancet Oncol. 2016, 17, 1158–1170.
- Kieffer, Y.; Hocine, H.R.; Gentric, G.; Pelon, F.; Bernard, C.; Bourachot, B.; Lameiras, S.; Albergante, L.; Bonneau, C.; Guyard, A.; et al. Single-Cell Analysis Reveals Fibroblast Clusters Linked to Immunotherapy Resistance in Cancer. Cancer Discov. 2020, 10, 1330–1351.
- Wang, W.; Kryczek, I.; Dostál, L.; Lin, H.; Tan, L.; Zhao, L.; Lu, F.; Wei, S.; Maj, T.; Peng, D.; et al. Effector T Cells Abrogate Stroma-Mediated Chemoresistance in Ovarian Cancer. Cell 2016, 165, 1092–1105.
- Hesler, R.A.; Huang, J.J.; Starr, M.D.; Treboschi, V.M.; Bernanke, A.G.; Nixon, A.B.; McCall, S.J.; White, R.R.; Blobe, G.C. TGF-β-induced stromal CYR61 promotes resistance to gemcitabine in pancreatic ductal adenocarcinoma through downregulation of the nucleoside transporters hENT1 and hCNT3. Carcinogenesis 2016, 37, 1041–1051.
- Su, S.; Chen, J.; Yao, H.; Liu, J.; Yu, S.; Lao, L.; Wang, M.; Luo, M.; Xing, Y.; Chen, F.; et al. CD10(+)GPR77(+) Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell 2018, 172, 841–856.e816.
- Kinugasa, Y.; Matsui, T.; Takakura, N. CD44 expressed on cancer-associated fibroblasts is a functional molecule supporting the stemness and drug resistance of malignant cancer cells in the tumor microenvironment. Stem Cells 2014, 32, 145–156.
- Jiang, Y.; Sun, L.; Zhang, Y.; Chen, Y.; Zhang, X.; Yan, S.; Xiao, L. The study of cancer-associated fibroblasts in cisplatin resistance of human ovarian cancer cells. Acta Univ. Med. Anhui 2021, 56, 255–260. (In Chinese)
- Au Yeung, C.L.; Co, N.-N.; Tsuruga, T.; Yeung, T.-L.; Kwan, S.-Y.; Leung, C.S.; Li, Y.; Lu, E.S.; Kwan, K.; Wong, K.-K.; et al. Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat. Commun. 2016, 7, 11150.
- Jiang, H.W.; Li, L.; Jiang, P.; Wang, Y.F. MicroRNA-489 targets XIAP to inhibit the biological progression of ovarian cancer via regulating PI3K/Akt signaling pathway and epithelial-to-mesenchymal transition. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4113–4122.
- Pfisterer, J.; Shannon, C.M.; Baumann, K.; Rau, J.; Harter, P.; Joly, F.; Sehouli, J.; Canzler, U.; Schmalfeldt, B.; Dean, A.P.; et al. Bevacizumab and platinum-based combinations for recurrent ovarian cancer: A randomised, open-label, phase 3 trial. Lancet Oncol. 2020, 21, 699–709.
- Pfisterer, J.; Plante, M.; Vergote, I.; du Bois, A.; Hirte, H.; Lacave, A.J.; Wagner, U.; Stähle, A.; Stuart, G.; Kimmig, R.; et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: An intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J. Clin. Oncol. 2006, 24, 4699–4707.
- Herrera, F.G.; Ronet, C.; Ochoa de Olza, M.; Barras, D.; Crespo, I.; Andreatta, M.; Corria-Osorio, J.; Spill, A.; Benedetti, F.; Genolet, R.; et al. Low-Dose Radiotherapy Reverses Tumor Immune Desertification and Resistance to Immunotherapy. Cancer Discov. 2022, 12, 108–133.
- Steer, A.; Cordes, N.; Jendrossek, V.; Klein, D. Impact of Cancer-Associated Fibroblast on the Radiation-Response of Solid Xenograft Tumors. Front. Mol. Biosci. 2019, 6, 70.
- Mantoni, T.S.; Lunardi, S.; Al-Assar, O.; Masamune, A.; Brunner, T.B. Pancreatic stellate cells radioprotect pancreatic cancer cells through β1-integrin signaling. Cancer Res. 2011, 71, 3453–3458.
- Al-Assar, O.; Demiciorglu, F.; Lunardi, S.; Gaspar-Carvalho, M.M.; McKenna, W.G.; Muschel, R.M.; Brunner, T.B. Contextual regulation of pancreatic cancer stem cell phenotype and radioresistance by pancreatic stellate cells. Radiother. Oncol. 2014, 111, 243–251.
- Theys, J.; Jutten, B.; Habets, R.; Paesmans, K.; Groot, A.J.; Lambin, P.; Wouters, B.G.; Lammering, G.; Vooijs, M. E-Cadherin loss associated with EMT promotes radioresistance in human tumor cells. Radiother. Oncol. 2011, 99, 392–397.
- Feig, C.; Jones, J.O.; Kraman, M.; Wells, R.J.B.; Deonarine, A.; Chan, D.S.; Connell, C.M.; Roberts, E.W.; Zhao, Q.; Caballero, O.L.; et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 20212–20217.
- Herrera, F.G.; Irving, M.; Kandalaft, L.E.; Coukos, G. Rational combinations of immunotherapy with radiotherapy in ovarian cancer. Lancet Oncol. 2019, 20, e417–e433.
- Rocconi, R.P.; Grosen, E.A.; Ghamande, S.A.; Chan, J.K.; Barve, M.A.; Oh, J.; Tewari, D.; Morris, P.C.; Stevens, E.E.; Bottsford-Miller, J.N.; et al. Gemogenovatucel-T (Vigil) immunotherapy as maintenance in frontline stage III/IV ovarian cancer (VITAL): A randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Oncol. 2020, 21, 1661–1672.
- Yousefi, H.; Momeny, M.; Ghaffari, S.H.; Parsanejad, N.; Poursheikhani, A.; Javadikooshesh, S.; Zarrinrad, G.; Esmaeili, F.; Alishahi, Z.; Sabourinejad, Z.; et al. IL-6/IL-6R pathway is a therapeutic target in chemoresistant ovarian cancer. Tumori 2019, 105, 84–91.
- Deying, W.; Feng, G.; Shumei, L.; Hui, Z.; Ming, L.; Hongqing, W. CAF-derived HGF promotes cell proliferation and drug resistance by up-regulating the c-Met/PI3K/Akt and GRP78 signalling in ovarian cancer cells. Biosci. Rep. 2017, 37, BSR20160470.
- Sun, Y.; Fan, X.; Zhang, Q.; Shi, X.; Xu, G.; Zou, C. Cancer-associated fibroblasts secrete FGF-1 to promote ovarian proliferation, migration, and invasion through the activation of FGF-1/FGFR4 signaling. Tumour. Biol. 2017, 39, 1010428317712592.
- Desbois, M.; Udyavar, A.R.; Ryner, L.; Kozlowski, C.; Guan, Y.; Dürrbaum, M.; Lu, S.; Fortin, J.P.; Koeppen, H.; Ziai, J.; et al. Integrated digital pathology and transcriptome analysis identifies molecular mediators of T-cell exclusion in ovarian cancer. Nat. Commun. 2020, 11, 5583.
- Affo, S.; Yu, L.X.; Schwabe, R.F. The Role of Cancer-Associated Fibroblasts and Fibrosis in Liver Cancer. Annu. Rev. Pathol. 2017, 12, 153–186.
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E.; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548.
- Walter, A.; Rocconi, R.P.; Monk, B.J.; Herzog, T.J.; Manning, L.; Bognar, E.; Wallraven, G.; Aaron, P.; Horvath, S.; Tang, M.; et al. Gemogenovatucel-T (Vigil) maintenance immunotherapy: 3-year survival benefit in homologous recombination proficient (HRP) ovarian cancer. Gynecol. Oncol. 2021, 163, 459–464.
- Sui, H.; Shi, C.; Yan, Z.; Li, H. Combination of erlotinib and a PARP inhibitor inhibits growth of A2780 tumor xenografts due to increased autophagy. Drug Des. Dev. Ther. 2015, 9, 3183–3190.
- Zhao, X.; Fang, Y.; Yang, Y.; Qin, Y.; Wu, P.; Wang, T.; Lai, H.; Meng, L.; Wang, D.; Zheng, Z.; et al. Elaiophylin, a novel autophagy inhibitor, exerts antitumor activity as a single agent in ovarian cancer cells. Autophagy 2015, 11, 1849–1863.
- Zalcman, G.; Mazieres, J.; Margery, J.; Greillier, L.; Audigier-Valette, C.; Moro-Sibilot, D.; Molinier, O.; Corre, R.; Monnet, I.; Gounant, V.; et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A randomised, controlled, open-label, phase 3 trial. Lancet 2016, 387, 1405–1414.
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011, 365, 2484–2496.
- Kraman, M.; Bambrough, P.J.; Arnold, J.N.; Roberts, E.W.; Magiera, L.; Jones, J.O.; Gopinathan, A.; Tuveson, D.A.; Fearon, D.T. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 2010, 330, 827–830.
- Lai, D.; Ma, L.; Wang, F. Fibroblast activation protein regulates tumor-associated fibroblasts and epithelial ovarian cancer cells. Int. J. Oncol. 2012, 41, 541–550.
- Li, J.; Liu, X.; Zang, S.; Zhou, J.; Zhang, F.; Sun, B.; Qi, D.; Li, X.; Kong, J.; Jin, D.; et al. Small extracellular vesicle-bound vascular endothelial growth factor secreted by carcinoma-associated fibroblasts promotes angiogenesis in a bevacizumab-resistant manner. Cancer Lett. 2020, 492, 71–83.
- Mitra, A.K.; Zillhardt, M.; Hua, Y.; Tiwari, P.; Murmann, A.E.; Peter, M.E.; Lengyel, E. MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer Discov. 2012, 2, 1100–1108.
- Li, X.; Fang, T.; Xu, S.; Jin, P.; Zhou, D.; Wang, Z.; Li, H.; Yang, Z.; Chen, G.; Zheng, X.; et al. PARP inhibitors promote stromal fibroblast activation by enhancing CCL5 autocrine signaling in ovarian cancer. NPJ Precis. Oncol. 2021, 5, 49.
- Kojima, Y.; Acar, A.; Eaton, E.N.; Mellody, K.T.; Scheel, C.; Ben-Porath, I.; Onder, T.T.; Wang, Z.C.; Richardson, A.L.; Weinberg, R.A.; et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc. Natl. Acad. Sci. USA 2010, 107, 20009–20014.
- Yeung, T.L.; Leung, C.S.; Wong, K.K.; Samimi, G.; Thompson, M.S.; Liu, J.; Zaid, T.M.; Ghosh, S.; Birrer, M.J.; Mok, S.C. TGF-β modulates ovarian cancer invasion by upregulating CAF-derived versican in the tumor microenvironment. Cancer Res. 2013, 73, 5016–5028.
- Jiménez-Sánchez, A.; Cybulska, P.; Mager, K.L.; Koplev, S.; Cast, O.; Couturier, D.L.; Memon, D.; Selenica, P.; Nikolovski, I.; Mazaheri, Y.; et al. Unraveling tumor-immune heterogeneity in advanced ovarian cancer uncovers immunogenic effect of chemotherapy. Nat. Genet. 2020, 52, 582–593.
- Sherman, M.H.; Yu, R.T.; Engle, D.D.; Ding, N.; Atkins, A.R.; Tiriac, H.; Collisson, E.A.; Connor, F.; Van Dyke, T.; Kozlov, S.; et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014, 159, 80–93.
- Froeling, F.E.M.; Feig, C.; Chelala, C.; Dobson, R.; Mein, C.E.; Tuveson, D.A.; Clevers, H.; Hart, I.R.; Kocher, H.M. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-β-catenin signaling to slow tumor progression. Gastroenterology 2011, 141, 1486–1497.e14.
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567.
- Desbois, M.; Wang, Y. Cancer-associated fibroblasts: Key players in shaping the tumor immune microenvironment. Immunol. Rev. 2021, 302, 241–258.
- Givel, A.M.; Kieffer, Y.; Scholer-Dahirel, A.; Sirven, P.; Cardon, M.; Pelon, F.; Magagna, I.; Gentric, G.; Costa, A.; Bonneau, C.; et al. miR200-regulated CXCL12β promotes fibroblast heterogeneity and immunosuppression in ovarian cancers. Nat. Commun. 2018, 9, 1056.
- Browning, L.; Patel, M.R.; Horvath, E.B.; Tawara, K.; Jorcyk, C.L. IL-6 and ovarian cancer: Inflammatory cytokines in promotion of metastasis. Cancer Manag. Res. 2018, 10, 6685–6693.
- Szulc-Kielbik, I.; Kielbik, M.; Nowak, M.; Klink, M. The implication of IL-6 in the invasiveness and chemoresistance of ovarian cancer cells. Systematic review of its potential role as a biomarker in ovarian cancer patients. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188639.
- Zhang, H.; Jiang, R.; Zhou, J.; Wang, J.; Xu, Y.; Zhang, H.; Gu, Y.; Fu, F.; Shen, Y.; Zhang, G.; et al. CTL Attenuation Regulated by PS1 in Cancer-Associated Fibroblast. Front. Immunol. 2020, 11, 999.
- Turcotte, M.; Spring, K.; Pommey, S.; Chouinard, G.; Cousineau, I.; George, J.; Chen, G.M.; Gendoo, D.M.; Haibe-Kains, B.; Karn, T.; et al. CD73 is associated with poor prognosis in high-grade serous ovarian cancer. Cancer Res. 2015, 75, 4494–4503.
- Koliaraki, V.; Pallangyo, C.K.; Greten, F.R.; Kollias, G. Mesenchymal Cells in Colon Cancer. Gastroenterology 2017, 152, 964–979.
- Steele, N.G.; Biffi, G.; Kemp, S.B.; Zhang, Y.; Drouillard, D.; Syu, L.; Hao, Y.; Oni, T.E.; Brosnan, E.; Elyada, E.; et al. Inhibition of Hedgehog Signaling Alters Fibroblast Composition in Pancreatic Cancer. Clin. Cancer Res. 2021, 27, 2023–2037.
This entry is offline, you can click here to edit this entry!
