A universal infrastructural issue is wetting of surfaces; millions of dollars are invested annually for rehabilitation and maintenance of infrastructures including roadways and buildings to fix the damages caused by moisture and frost. The biomimicry of the lotus leaf can provide superhydrophobic surfaces that can repel water droplets, thus reducing the penetration of moisture, which is linked with many deterioration mechanisms in infrastructures, such as steel corrosion, sulfate attack, alkali-aggregate reactions, and freezing and thawing. In cold-region countries, the extent of frost damage due to freezing of moisture in many components of infrastructures will be decreased significantly if water penetration can be minimized. Consequently, it will greatly reduce the maintenance and rehabilitation costs of infrastructures.
- biomimetic coating
- durability
- infrastructures
- lotus leaf
- self-cleaning
- superhydrophobicity
1. Introduction
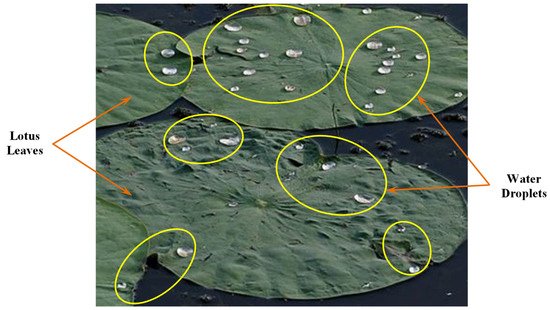
2. Surface Structure and Characteristics of Lotus Leaf
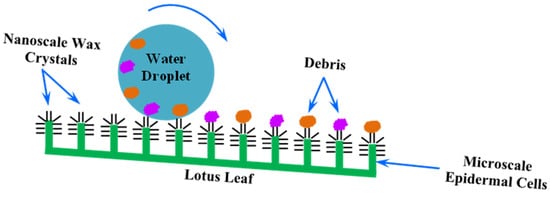

3. Lotus-Leaf-Inspired Biomimetic Coatings
| Coating Material | Key Characteristics | Specific Purposes | References |
|---|---|---|---|
| PDMS (Polydimethylsiloxane) | Intrinsic hydrophobic surface; remarkably high contact angle (close to 170°); sliding angle close to that of the lotus leaf; highly water-resistant; good self-cleaning property; chemically and thermally stable; stretchable. | Inverse-trapezoidal microstructures; microfabrication with micropillars/nanohairs. | [32,33,34,35] |
| UPC (Ultrafine powder coating) | At 3% PTFE (polytetrafluoroethylene): High contact angle (˃160°) and low sliding angle (˂5°); lower film thickness (controllable to 1 mil); reduced surface roughness; high-quality surface finishing. | Surface protection from moisture intervention | [13,36] |
| CNT (Carbon nanotube) film | Excellent anti-aging performance; effective to prevent the penetration of small water droplets; long-term durability after exposure to air and corrosive liquids. | Electrodes, biosensors, anti-fogging/icing and anti-aging materials. | [37,38] |
| Nickel (Ni), Ni/Nano-C, Ni/Nano-Cu | PFPE (perfluoropolyether) treated Ni: high contact angle (156°) and a rough surface; reduced friction coefficient; high hardness. Ni/Nano-C (Ni-C): better anti-corrosion performance. Ni/Nano-Cu (Ni-Cu): Large contact angle (155.5°) at optimal brush speed and a Cu concentration of 5 g/L; a sliding angle of 5°. |
Substrate protection; anti-corrosion surface coatings. | [39,40] |
| FOTS-TiO2 (Fluoro-octyl-trichloro-silane-titanium) | Superamphiphobic (superhydro-oleophobic); high contact angle with peanut oil; liquid repellence with a surface tension as low as 23.8 mN/m; high thermal stability; self-cleaning; anti-fouling/anti-icing. | Surface treatment of materials and products (Zn plate, PU sponge, filter paper, cotton fibers, etc.); civil infrastructure maintenance; temperature sensitive nanotechnology applications. | [31,41] |
| Janus particles | Superhydrophobic performance with nanoscale roughness; covalent binding with substrate; tolerant to high water flushing speeds and organic solvents. | Nanoprobes, nanosensors, display systems, water-repellent textiles, drug delivery and control systems, functional coatings, etc. | [42,43] |
| Diamond-like carbon (DLC) | Balance of hardness and flexibility due to microstructures; high contact angle; low friction coefficient; greater corrosion resistance. | Bio-robotics, bio-medical devices, anti-corrosion surface coatings. | [44,45,46] |
| Micro- and nanosized silica (SiO2) particles | Strong liquid (e.g., water, brine, acidic solution) repellency with a high contact angle and a low sliding angle for droplets; strong binding adhesion with underlying substrate; high weathering resistance including UV (ultraviolet) protection; high transmission of light with low reflection; excellent wear and scratch resistance. | Anti-abrasion, anti-corrosion, and waterproofing applications; surface coatings for self-cleaning and energy harvesting. | [47,48] |
| Calcium hydroxide [Ca(OH)2] microcapsules with polymeric shell | Regenerative lotus effect—controllable via sodium stearate solution; good resistance to water flushing; strong binding adhesion to substrate; superior corrosion resistance in chloride environment. | Substrate modifications, corrosion-resistant coatings. | [49,50] |
| Graphene oxide-silica (GO-SiO2) | Highly hydrophilic; superior barrier performance and corrosion protection; good binding adhesion with substrate. | Electrode, capacitor, and biosensor fabrication; anti-corrosion composite coatings. | [51,52,53] |
| Photopolymer (PP) | Transparent; anti-reflective and self-cleaning abilities; increased solar light absorbance; UV- or electron-beam curable; resistant to acidic and basic conditions. | Harvesting of alternative energy—coating on solar cells; protective coatings and decorative finishes; surface modifications of fibers and films; coatings for biosensors and electrodes. | [54,55] |
| Copper (Cu) | Superhydrophobic/superolephobic; hierarchical flowerlike surface morphology; long-term chemical stability; high contact angle for pure water as well as under both acidic and basic conditions. | Protection of steel surfaces; self-cleaning steel structures; oil pipelines for anti-fouling and low fluid drag. | [56,57] |
| Zinc oxide (ZnO) film | Can be either superhydrophobic or superhydrophilic depending on surface morphology; superhydrophobicity with a contact angle of 155° to more than 170°; superhydrophilicity with a low contact angle of approximately 1–2.8°; UV-stable. |
Self-cleaning PV (Photovoltaic) and glazing applications | [58,59,60] |
| Acrylic polymer (AP) | High water repellency; delayed ice nucleation; reduced binding adhesion with ice; lower freezing point of water. | Anti-icing coatings for pavement/building protection from frost damage; anti/de-icing systems for cars and airplanes, telecommunication antennas, or wind turbines. | [30,61] |
| Antimony doped tin oxide/polyurethane (ATO/PU) film | Superhydrophobicity and high heat-insulation; water contact angle up to about 155°; high visible light transmittance (76%); low infrared transmission; high thermal stability. | Self-cleaning solar cells; heat-insulating glass. | [62,63,64] |
| PMMA (Polymethyl methacrylate) | Increased PV efficiency (up to 17% gains); high optical transparency (˃80%); low reflection; chemically resistant to aqueous alkalis and most acids; high moisture resistance; protected from oxygen; UV-durable; abrasion-resistant. | Natural light harvesting for alternative energy; roofing membranes; balcony and parking deck surfacing and waterproofing applications. | [65,66] |
| PPS/PTFE (Polyphenylene sulfide/polytetrafluoroethylene) | Superamphiphobic; high contact angle (151–172°); excellent impact and wear resistance; low coefficient of friction; high cohesion and thermal stability; high anti-scaling ability. | Lubricant surface coatings; anti-scaling coatings. | [67,68,69,70] |
This entry is adapted from the peer-reviewed paper 10.3390/infrastructures7040046
