Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Pumpkin is a well-known multifunctional ingredient in the diet, full of nutrients, and has opened new vistas for scientists during the past years. The fruit of pumpkin including the flesh, seed, and peel are a rich source of primary and secondary metabolites, including proteins, carbohydrates, monounsaturated fatty acids, polyunsaturated fatty acids, carotenoids, tocopherols, tryptophan, delta-7-sterols, and many other phytochemicals.
- pumpkin
- phytochemicals
- bioactive compounds
- therapeutic potential
1. Introduction
Products from natural sources have been used for centuries as functional and nutraceutical foods [1][2][3][4][5][6]. During the past years, scientists have been working on understanding the molecular-level effects of various nutrients on several chronic and deadly diseases [7][8][9]. People can adjust to different climate and habitat changes due to a wide range of nutrients that alter multiple genes’ natural mode at the molecular level [10]. In other words, molecular changes are due to nutrients. Keeping this in mind, researchers are engaged in preventing and treating diseases with the use of pertinent food(s) rather than medicines. Moreover, studies have demonstrated that healthy eating is a reasonable and economical method to treat diseases [11].
Pumpkin has attracted increasing attention from scientists due to its nutritional profile. It is a nutritious and economical product and belongs to the Cucurbitaceae family. Cucurbita pepo L., Cucurbita maxima Duchesne, and Cucurbita moschata Duchesne are harvested worldwide due to their economical and environmentally friendly properties [12]. In many countries, pumpkin is used as a medicine for its anti-inflammatory, antioxidant, antiviral, and antidiabetic properties, particularly in Austria, Hungary, Mexico, Slovenia, China, Spain, and various European, Asian, and African countries [13]. Worldwide, pumpkin is harvested for its peel, flesh, and seeds. The seeds are usually large in size with a high content of polyunsaturated and monounsaturated fatty acids. Linoleic acid, oleic acid, palmitic acid, ECN-44, ECN-46, tocopherols, ß-sitosterol, and delta-7-sterols constitute a large quantity of pumpkin seed oil [14].
For decades, several research studies have been conducted on the active ingredients of pumpkin peel, flesh, and seeds to provide a thumbnail sketch of their health-related impacts, which have demonstrated its anti-inflammatory [15], antibacterial [16], anticarcinogenic [17], antidiabetic [18], and antihypertensive properties, associated with this climber for diabetes [19].
Figure 1 illustrates the health-friendly properties of pumpkins.
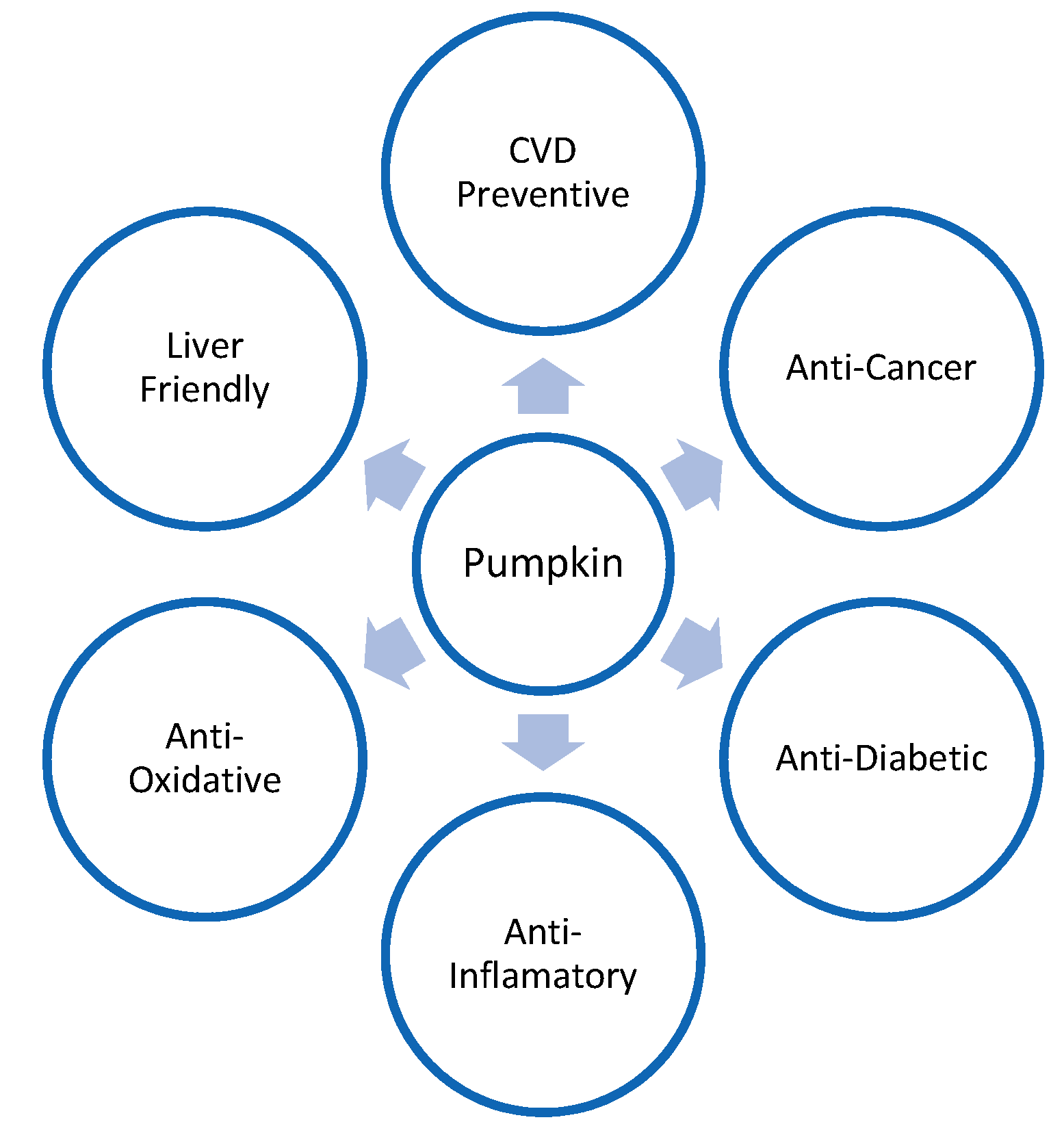
Figure 1. Health-friendly properties of pumpkin.
2. Health-Promoting Properties of Cucurbita
2.1. Hypoglycemic Properties
Hyperglycemia, which may result from the absence of insulin (DM-type 1) or due to a low response to insulin (DM-type 2), is one of the world’s emerging problems. Chronic hyperglycemia leads to severe complications, such as damage to the eyes (retinopathy), brain (neuropathy), and kidneys (nephropathy) [20]. Diabetes is remarkably spreading worldwide, suggesting a considerable rise to about 82 million sufferers by 2030 [21]. People in third world and middle-class countries suffer from DM as more than 80% of diabetes-associated deaths occur in these regions [22]. Several drugs, such as glucagon-like peptide-1 (GLP-1) analogs, α-glucosidase, metformin, etc. are used to cure DM-2. Nevertheless, these drugs have an effect on longevity, resulting in many adverse side effects. Due to the side effects of anti-hyperglycemic medicines, healthcare providers worldwide focus on the use of herbs and dietary ingredients to cure DM-2 [23].
In Mexico and China, herbal extracts are used to treat hyperglycemia as they usually contain pumpkin [24][25]. In recent years, extensive research has been conducted to study the antidiabetic effects of pumpkin flesh, seeds, and peel [26]. Pumpkin fruit powder was reported to contain antidiabetic properties [27]. In light of this study, it is reported that pumpkin powder tends to enhance the level of insulin in the body, leading to a lower level of glucose. Therefore, it also lowers the risk of kidney damage [28].
The breakdown of complex carbohydrates usually occurs in the small intestine by a-glycosidase, which is present in epithelial mucosa. A-glycosidase is responsible for the breakdown of glycosidic bonds present within complex carbs, and thus increases the blood glucose level [29]. It is often highlighted that the use of pumpkin can lower the activity of a-glycosidase. Moreover, in the same research, it has been reported that pumpkin scavenges ROS and can act as an ACE inhibitor [30]. However, further research is required to understand the effect of pumpkin on alpha-amylase.
Diabetic rats are one of the most commonly used tools to study the antidiabetic effect of any therapeutic agent. Alloxan is a toxin that affects the B-cells of pancreas, and thus leads to diabetes in rats [31]. A case-control study on rats demonstrates the anti-hyperglycemic properties of pumpkin. It further strengthens the research that pumpkin has the tendency to enhance insulin production and lowers the glucose level in the blood. The use of pumpkin in the earlier stages of diabetes may reduce postprandial glycemic levels. Protein-bound polysaccharide (PBPP) in pumpkin tends to lower hyperglycemia in rats and is usually dose-dependent [32]. In 2019, an in vivo study was conducted. Both pumpkin polysaccharide (PPe) and pumpkin polysaccharide hydrolysate (PPe-H) showed their hypoglycemic effect to lower fasting blood sugar levels of rats with DM-2. The diabetes-friendly nature of PPe-H has facilitated the decrease in oxidative stress and stimulated the endogenous GLP-1 secretion [33].
Figure 2 illustrates the mechanism of pumpkin polysaccharide hydrolysate in the treatment of DM-2.

Figure 2. Mechanism of pumpkin polysaccharide hydrolysate in the treatment of DM-2.
Pumpkin regulates the blood glucose level by promoting the discharge of insulin and preventing many of the complications associated with diabetes [34]. Taking this into account, it is stated that pumpkin has antidiabetic properties and can play a protective role against hyperglycemia in diabetic patients. Nevertheless, researchers could not use these data to successfully report the exact mechanism of action of pumpkin against diabetes.
2.2. Anti-Cancerous Properties
A diet rich in oxidants and antioxidants has a significant association with cancer. Diet tends to make the condition worse or better, as cancer is associated with oxidative stress [35]. In 2017, 9.6 million deaths were reported due to all types of cancers. A report by GLOBOCAN estimated that the number of individuals suffering from cancer in 2018 was 18.1 million, and the death rate will be 9.6 million in 2019 [36]. In less developed countries, the rate of stomach, cervical, and liver cancer is high, while in developed countries, breast, lung, and prostate cancer is more common [37][38].
Pumpkin seeds are a major source of phytoestrogen, such as lignans and isoflavones. Phytoestrogen tends to bind with estrogen receptors (ER) in the female body [39]. A study was conducted to investigate the association between breast cancer and pumpkin seed extract containing phytoestrogens. It was reported that the production of estradiol in MCF7, BeWo, and Jeg3 cells increased, and the reduction in ER-α was observed in MCF7-cells [40]. Essentially, 2S albumins which are proteins present within the seeds of pumpkin, have been reported in a study to have anti-cancerous properties, particularly in the case of breast cancer. MCF7-cells were treated with protein at two different concentrations (10 and 30 µM). The results obtained by DNA showed fragmentation assays and acridine orange staining, in which 2S albumins from pumpkin seeds have the tendency to cause apoptosis in MCF7-cell lines [41].
2.3. Neuroprotective Properties
Malnutrition is a common issue worldwide, affecting children with limited calorie and protein(s) intake. Malnutrition usually results in behavioral disorders [42]. It is reported that protein-energy malnutrition (PEM) gives rise to free radical production, usually through lipid peroxidation [43]. Lipid peroxidation is a risk factor associated with brain damage. Free radicals production, such as reactive oxygen species (ROS) damages the brain cells, resulting in severe harmful consequences of PEM [44][45]. In a recent study, the leaves of pumpkin (fluted pumpkin) were used to investigate the brain-protective effect of this herb in PEM-induced rats due to the high antioxidant composition [46]. The seed protein was clearly observed and fluted pumpkin leaves joined together to prevent oxidative damage to brain cells due to PEM.
Organic compounds known as aflatoxins have substantial toxic effects, such as carcinogenic, mutagenic, and hepatotoxic. In addition, they contribute to lipid peroxidation, thus affecting the brain [47]. In 2013, research was conducted to investigate the effect of pumpkin seed oil on aflatoxins-induced toxicity in the brain and other organs. It was reported that pumpkin seed oil has the tendency to treat aflatoxins-induced harmful effects in brain tissues [48].
Depression is the most common brain disorder and is specified as a state in which one loses interest, pleasure, sleep, and eating patterns are disturbed. Similarly, in mood swings, the sufferers may feel guilty and ashamed. In addition, they have poor interest/concentration in daily tasks. According to the 2015 World Health Organization statistics, people suffering from depression were more than 300 million [49]. In a study on fluted pumpkin leaves, it was reported that they can be a useful tool in treating depression and convulsions due to their muscle relaxant properties, particularly in hydroethanolic leaf extract [50]. Moreover, another report demonstrated the antidepressant effects of pumpkin in supporting the treatment of depression [51].
2.4. Liver Disease Preventive Properties
In the past, several researchers reported the liver-protective effects of pumpkin. In 2005, a study was conducted on male Sprague Dawley rats, which were administered a low protein diet for 5 days to produce liver dysfunction. Thereafter, they were administered CCl4 injections, which resulted in significantly higher levels of four liver enzymes; aspartate transaminase (AST), alkaline phosphatase (ALP), alanine transaminase (ALT), and lactate dehydrogenase (LD). One group was administered with pumpkin seed protein isolate, which lowered the level of both aforementioned enzymes, suggesting the beneficial role of pumpkin in treating liver dysregulation [52]. In a similar study which was carried out a year later (2006), the same results were reported in addition to antioxidative effects [53]. In another study performed on rats with CCl4, the same hepatoprotective effect of pumpkin seeds protein isolate was mainly reported by enhancing antioxidant activity and decreasing liver enzymes [54]. In a similar study, acetaminophen was used rather than CCl4 to produce liver injury, and the results were the same as reported earlier [55]. Another research, which was conducted to understand the effect of aqueous leaf extract of fluted pumpkin on anemia, also reported the same results. In this case, 50 mg/kg of aqueous leaf extract of fluted pumpkin has the tendency to regulate ALT and AST [56]. All of these researches support the fact that pumpkin seed protein isolates have the tendency to attenuate the high level of liver enzymes (ALT, AST, ALP, LD) when liver injury is due to a low protein diet or malnutrition. In 2015, Farid et al. reported the same alterations in these liver enzymes with the use of pumpkin [57].
Non-alcoholic fatty liver disease (NAFLD) further leads to atherosclerosis, and CVD is a major health issue worldwide that leads to mortality and morbidity [58]. To cure the NAFLD as well as the onset of NAFLD, intake of fats and the type of fat present in one’s diet play an important role [59][60]. As mentioned above, pumpkin seed oil is rich in unsaturated fatty acids that comprise about 80% of enriched phytochemicals [61].
2.5. CVD Preventive Properties
Pumpkin affects serum lipid levels directly and can also affect them indirectly. A research was conducted on Lohmann Brown Lite hens, and they were administered with a diet based on flaxseed and pumpkin oil. It was reported that eggs laid by the hens with a pumpkin diet had the highest MUFA content, lower PUFA, lower myristic acid, and other saturated fatty acids content [62]. However, further investigation is required as the earlier increase in myristic acid content was reported in eggs after pumpkin supplementation [63].
When the body is in hypercholesterolemic situation, dysfunctioning of the endothelial layer occurs, leading to the enhanced level of vascular cell adhesion molecule (VCAM). This occurs due to the activation of reactive oxygen species (ROS), which reduces nitric oxide formation and stops LDL oxidation. Due to the lower levels of nitric oxide (NO), oxidization of LDL will occur, which is responsible for all this VCAM enhancement mechanism [64]. Pumpkin seed powder contains 2.6% arginine, which is the precursor of NO, associated with BP maintenance, apoptosis, myocardial function, and inflammatory response [65]. An in vivo study designed to investigate the effect of pumpkin seed extract (mainly arginine) reported that pumpkin seed extract supplementation in rats with hyperlipidemia could enhance the expression of NO production due to the presence of arginine. Moreover, due to the production of NO, oxidation of LDL is attenuated, leading to the decreased expression of VCAM [66]. Therefore, it can be stated that modifying the lifestyle and adapting the regular use of pumpkins can be a useful dietary strategy to treat hypercholesterolemia.
2.6. Other Health-Related Properties
In addition to all of the aforementioned properties, pumpkin has been reported to play an important role in inflammatory diseases, such as arthritis due to its anti-inflammatory properties [67][68][69].
The worldwide male population above 50 years mostly suffers through an outgrowth of stromal and epithelial cells, usually in the periurethral area and in the transition zone [70][71]. Many researches support the claim that pumpkin seed extract has also been reported to overcome lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH), such as saw palmetto and prazosin [72][73]. In addition to treating LUTS, pumpkin administered for a whole month at a 14 mg/kg dose is a really useful strategy in controlling testosterone-induced hyperplasia [17]. Nevertheless, the data are not sufficient, and further researches are required as many consequences of pumpkin intake, in this case, are neglected in these researches.
Currently, although antimicrobial drugs are available, the focus of scientists is to develop dietary strategies to treat these infections as the use of these antimicrobes can lead to drug resistance. At a concentration of 2% (v/v), pumpkin seed oil has the tendency to treat Staphylococcus aureus, Salmonella enterica, Escherichia coli, Klebsiella pneumonia, Acinetobacter baumannii, Candida albicans, and Serratia marcescens [27]. Three hundred and seventy five milligrams of pumpkin seed oil can be useful to treat Mycosphaerella arachidis, Fusarium oxysporum, and Botrytis cinerea [74]. Similarly, proteins from pumpkin (>2 mM) in their purified form can control fungal growth [75]. Likewise, yeast growth has been known to be inhibited by three types of pumpkin proteins named MAP11, MAP4, and MAP2. However, MAP2 and MAP4 are not very useful in treating Gram-negative bacteria [76]. Therefore, pumpkin is a food of great antimicrobial properties and its use should be encouraged by people living in areas where they are more susceptible to any kind of microbial infection.
Pumpkin seeds have also shown anti-ulcerative properties by protecting gastric mucosa in a dose-dependent manner [77]. Wound healing through more effective and cost effective strategies is a constant focus of researchers, as medicines seem to be insufficient for the healing process [78][79]. High amounts of plant sterols, tocopherols, and PUFA present in pumpkin seed oil positively affect wound healing [14]. Similar findings that are related to pumpkin peel are also reported [80].
During the past years, male reproduction has been affected due to changes in lifestyle and some tragedies [81]. As pumpkins tend to attenuate lipid peroxidation and lower oxidative stress, it is a valuable tool to improve testicular properties and enhance reproduction [82][83]. A recent study conducted on fluted pumpkin seed (FPS) extract reported that 40 days of supplementation of 40 mg/kg bodyweight of FPS extract could enhance the number of spermatids, spermatocytes, and spermatogonia as well as protect against oxidative cell damage [84]. Therefore, FPS extract can be used as spermatocytes activator after chemotherapy. However, the effect is still uncertain, thus further investigation is required to understand the effect of pumpkin on serum parameters and testes [85].
This entry is adapted from the peer-reviewed paper 10.3390/plants11111394
References
- Ranjha, M.M.A.N.; Irfan, S.; Lorenzo, J.M.; Shafique, B.; Kanwal, R.; Pateiro, M.; Arshad, R.N.; Wang, L.; Nayik, G.A.; Roobab, U.; et al. Sonication, a Potential Technique for Extraction of Phytoconstituents: A Systematic Review. Processes 2021, 9, 1406.
- Ranjha, M.M.A.N.; Kanwal, R.; Shafique, B.; Arshad, R.N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P.Ł.; Irfan, M.; Khalid, M.Z.; Roobab, U.; et al. A Critical Review on Pulsed Electric Field: A Novel Technology for the Extraction of Phytoconstituents. Molecules 2021, 26, 4893.
- Nadeem, H.; Akhtar, S.; Ismail, T.; Sestili, P.; Lorenzo, J.; Ranjha, M.; Jooste, L.; Hano, C.; Aadil, R. Heterocyclic Aromatic Amines in Meat: Formation, Isolation, Risk Assessment, and Inhibitory Effect of Plant Extracts. Foods 2021, 10, 1466.
- Ranjha, M.M.A.N.; Shafique, B.; Wang, L.; Irfan, S.; Safdar, M.N.; Murtaza, M.A.; Nadeem, M.; Mahmood, S.; Mueen-Ud-Din, G.; Nadeem, H.R. A comprehensive review on phytochemistry, bioactivity and medicinal value of bioactive compounds of pomegranate (Punica granatum). Adv. Tradit. Med. 2021, 1–21.
- Ranjha, M.M.A.N.; Amjad, S.; Ashraf, S.; Khawar, L.; Safdar, M.N.; Jabbar, S.; Nadeem, M.; Mahmood, S.; Murtaza, M.A. Extraction of Polyphenols from Apple and Pomegranate Peels Employing Different Extraction Techniques for the Development of Functional Date Bars. Int. J. Fruit Sci. 2020, 20, S1201–S1221.
- Ranjha, M.M.A.N.; Irfan, S.; Nadeem, M.; Mahmood, S. A Comprehensive Review on Nutritional Value, Medicinal Uses, and Processing of Banana. Food Rev. Int. 2020, 38, 199–225.
- Rasheed, H.; Shehzad, M.; Rabail, R.; Kowalczewski, P.; Kidoń, M.; Jeżowski, P.; Ranjha, M.M.A.N.; Rakha, A.; Din, A.; Aadil, R.M. Delving into the Nutraceutical Benefits of Purple Carrot against Metabolic Syndrome and Cancer: A Review. Appl. Sci. 2022, 12, 3170.
- Khalid, W.; Gill, P.; Arshad, M.S.; Ali, A.; Ranjha, M.M.A.N.; Mukhtar, S.; Afzal, F.; Maqbool, Z. Functional Behavior of DHA and EPA in the Formation of Babies Brain at Different Stages of Age, and Protect from Different Brain-Related Diseases. Int. J. Food Prop. 2022, 25, 1021–1044.
- Mutch, D.; Wahli, W.; Williamson, G. Nutrigenomics and Nutrigenetics: The Emerging Faces of Nutrition. FASEB J. 2005, 19, 1602–1616.
- Rotilio, G.; Marchese, E. Nutritional Factors in Human Dispersals. Ann. Hum. Biol. 2010, 37, 312–324.
- Brunner, E.; Cohen, D.; Toon, L. Cost Effectiveness of Cardiovascular Disease Prevention Strategies: A Perspective on EU Food Based Dietary Guidelines. Public Health Nutr. 2001, 4, 711–715.
- Caili, F.; Huan, S.; Quanhong, L. A Review on Pharmacological Activities and Utilization Technologies of Pumpkin. Mater. Veg. 2006, 61, 70–77.
- Ayyildiz, H.F.; Topkafa, M.; Kara, H. Pumpkin (Cucurbita pepo L.) Seed Oil. In Fruit Oils: Chemistry and Functionality; Ramadan, M.F., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 765–788. ISBN 978-3-030-12473-1.
- Bardaa, S.; Ben Halima, N.; Aloui, F.; Ben Mansour, R.; Jabeur, H.; Bouaziz, M.; Sahnoun, Z. Oil from pumpkin (Cucurbita pepo L.) seeds: Evaluation of its functional properties on wound healing in rats. Lipids Health Dis. 2016, 15, 1–12.
- Nawirska-Olszańska, A.; Biesiada, A.; Sokół-Łętowska, A.; Kucharska, A.Z. Characteristics of organic acids in the fruit of different pumpkin species. Food Chem. 2014, 148, 415–419.
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial Activity of Essential Oils and Other Plant Extracts. J. Appl. Microbiol. 1999, 86, 985–990.
- Gossell-Williams, M.; Davis, A.; O′connor, N. Inhibition of Testosterone-Induced Hyperplasia of the Prostate of Sprague-Dawley Rats by Pumpkin Seed Oil. J. Med. Food 2006, 9, 284–286.
- Boaduo, N.K.K.; Katerere, D.; Eloff, J.N.; Naidoo, V. Evaluation of Six Plant Species Used Traditionally in the Treatment and Control of Diabetes Mellitus in South Africa Using in Vitro Methods. Pharm. Biol. 2014, 52, 756–761.
- Alshammari, G.M.; Balakrishnan, A. Pumpkin (Cucurbita ficifolia Bouché) Extract Attenuate the Adipogenesis in Human Mesenchymal Stem Cells by Controlling Adipogenic Gene Expression. Saudi J. Biol. Sci. 2019, 26, 744–751.
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2014, 37, S81–S90.
- Wild, S.H.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030. Diabetes Care 2004, 27, 1047–1053.
- Xu, X.; Shan, B.; Liao, C.-H.; Xie, J.-H.; Wen, P.-W.; Shi, J.-Y. Anti-Diabetic Properties of Momordica Charantia L. Polysaccharide in Alloxan-Induced Diabetic Mice. Int. J. Biol. Macromol. 2015, 81, 538–543.
- Zhang, C.; Chen, H.; Bai, W. Characterization of Momordica Charantia L. Polysaccharide and Its Protective Effect on Pancreatic Cells Injury in STZ-Induced Diabetic Mice. Int. J. Biol. Macromol. 2018, 115, 45–52.
- Andrade-Cetto, A.; Heinrich, M. Mexican Plants with Hypoglycaemic Effect Used in the Treatment of Diabetes. J. Ethnopharmacol. 2005, 99, 325–348.
- Jia, W.; Gaoz, W.; Tang, L. Antidiabetic Herbal Drugs Officially Approved in China. Phyther. Res. 2003, 17, 1127–1134.
- Rolnik, A.; Olas, B. Vegetables from the Cucurbitaceae family and their products: Positive effect on human health. Nutrition 2020, 78, 110788.
- Ahmad, G.; Khan, A.A. Pumpkin: Horticultural Importance and Its Roles in Various Forms; a Review. Int. J. Hortic. Agric. 2019, 4, 1–6.
- Chen, J.G.; Liu, Z.Q.; Wang, Y.; Lai, W.Q.; Mei, S.; Fu, Y. Effects of Sugar-Removed Pumpkin Zymptic Powders in Preventing and Treating the Increase of Blood Glucose in Alloxan-Induced Diabetic Mice. Chin. J. Clin. Rehabil. 2005, 9, 94–95.
- Jaiswal, N.; Srivastava, S.P.; Bhatia, V.; Mishra, A.; Sonkar, A.K.; Narender, T.; Srivastava, A.K.; Tamrakar, A.K. Inhibition of Alpha-Glucosidase by Acacia Nilotica Prevents Hyperglycemia along with Improvement of Diabetic Complications via Aldose Reductase Inhibition. J. Diabetes Metab. 2012, 6, 7.
- Kwon, Y.-I.; Apostolidis, E.; Kim, Y.-C.; Shetty, K. Health Benefits of Traditional Corn, Beans, and Pumpkin: In Vitro Studies for Hyperglycemia and Hypertension Management. J. Med. Food 2007, 10, 266–275.
- Bonner-Weir, S.; Weir, G.C. New Sources of Pancreatic β-Cells. Nat. Biotechnol. 2005, 23, 857.
- Quanhong, L.; Caili, F.; Yukui, R.; Guanghui, H.; Tongyi, C. Effects of Protein-Bound Polysaccharide Isolated from Pumpkin on Insulin in Diabetic Rats. Mater. Veg. 2005, 60, 13–16.
- Chen, X.; Qian, L.; Wang, B.; Zhang, Z.; Liu, H.; Zhang, Y.; Liu, J. Synergistic Hypoglycemic Effects of Pumpkin Polysaccharides and Puerarin on Type II Diabetes Mellitus Mice. Molecules 2019, 24, 955.
- Askari, G.; Bayat, A.; Azizi-Soleiman, F.; Heidari-Beni, M.; Feizi, A.; Iraj, B.; Ghiasvand, R. Effect of cucurbita ficifolia and probiotic yogurt consumption on blood glucose, lipid profile, and inflammatory marker in Type 2 Diabetes. Int. J. Prev. Med. 2016, 7, 30.
- Halliwell, B.; Zhao, K.; Whiteman, M. The Gastrointestinal Tract: A Major Site of Antioxidant Action? Free Radic. Res. 2000, 33, 819–830.
- Khazaei, Z.; Jarrahi, A.M.; Momenabadi, V.; Ghorat, F.; Adineh, H.A.; Sohrabivafa, M.; Goodarzi, E. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide Stomach Cancers and Their Relationship with the Human Development Index (HDI). World Cancer Res. J. 2019, 6, e1257.
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108.
- WHO. Health Statistics and Information Systems: WHO Mortality Database. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates (accessed on 23 March 2022).
- Velentzis, L.S.; Woodside, J.V.; Cantwell, M.M.; Leathem, A.J.; Keshtgar, M.R. Do Phytoestrogens Reduce the Risk of Breast Cancer and Breast Cancer Recurrence? What Clinicians Need to Know. Eur. J. Cancer 2008, 44, 1799–1806.
- Richter, D.; Abarzua, S.; Chrobak, M.; Vrekoussis, T.; Weissenbacher, T.; Kühn, C.; Schulze, S.; Kupka, M.S.; Friese, K.; Briese, V.; et al. Effects of Phytoestrogen Extracts Isolated from Pumpkin Seeds on Estradiol Production and ER/PR Expression in Breast Cancer and Trophoblast Tumor Cells. Nutr. Cancer 2013, 65, 739–745.
- Tomar, P.P.S.; Nikhil, K.; Singh, A.; Selvakumar, P.; Roy, P.; Sharma, A.K. Characterization of Anticancer, DNase and Antifungal Activity of Pumpkin 2S Albumin. Biochem. Biophys. Res. Commun. 2014, 448, 349–354.
- Bonatto, F.; Polydoro, M.; Andrades, M.; Júnior, M.L.C.D.F.; Pizzol, F.D.; Rotta, L.; Souza, D.; Perry, M.L.; Moreira, J.C.F. Effects of maternal protein malnutrition on oxidative markers in the young rat cortex and cerebellum. Neurosci. Lett. 2006, 406, 281–284.
- Kayode, O.; Kayode, A.; Odetola, A. Therapeutic Effect of Telfairia Occidentalis on Protein Energy Malnutrition-Induced Liver Damage. Res. J. Med. Plant 2009, 3, 80–92.
- Potukuchi, A.; Addepally, U.; Sindhu, K.; Manchala, R. Increased Total DNA Damage and Oxidative Stress in Brain Are Associated with Decreased Longevity in High Sucrose Diet Fed WNIN/Gr-Ob Obese Rats. Nutr. Neurosci. 2017, 21, 648–656.
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84.
- Kayode, A.; Kayode, O.; Odetola, A. Telfairia occidentalis Ameliorates Oxidative Brain Damage in Malnorished Rats. Int. J. Biol. Chem. 2009, 4, 10–18.
- Paterson, R.R.M.; Lima, N. Toxicology of Mycotoxins. In Molecular, Clinical and Environmental Toxicology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 31–63.
- Eraslan, G.; Kanbur, M.; Aslan, Ö.; Karabacak, M. The Antioxidant Effects of Pumpkin Seed Oil on Subacute Aflatoxin Poisoning in Mice. Environ. Toxicol. 2013, 28, 681–688.
- Kessler, R.C.; A Sampson, N.; Berglund, P.; Gruber, M.J.; Al-Hamzawi, A.; Andrade, L.; Bunting, B.; Demyttenaere, K.; Florescu, S.; De Girolamo, G.; et al. Anxious and non-anxious major depressive disorder in the World Health Organization World Mental Health Surveys. Epidemiol. Psychiatr. Sci. 2015, 24, 210–226.
- Akindele, A.J.; Ajao, M.Y.; Aigbe, F.R.; Enumah, U.S. Effects of Telfairia Occidentalis (Fluted Pumpkin; Cucurbitaceae) in Mouse Models of Convulsion, Muscle Relaxation, and Depression. J. Med. Food 2013, 16, 810–816.
- Patel, S. Pumpkin (Cucurbita sp.) Seeds as Nutraceutic: A Review on Status Quo and Scopes. Mediterr. J. Nutr. Metab. 2013, 6, 183–189.
- Nkosi, C.Z.; Opoku, A.R.; Terblanche, S.E. Effect of pumpkin seed (Cucurbita pepo) protein isolate on the activity levels of certain plasma enzymes in CCl4-induced liver injury in low-protein fed rats. Phytother. Res. 2005, 19, 341–345.
- Nkosi, C.Z.; Opoku, A.R.; Terblanche, S.E. Antioxidative effects of pumpkin seed (Cucurbita pepo) protein isolate in CCl4-Induced liver injury in Low-Protein fed rats. Phytother. Res. 2006, 20, 935–940.
- Mohamed, R.A.; Ramadan, R.S.; Ahmed, L.A. Effect of Substituting Pumpkin Seed Protein Isolate for Casein on Serum Liver Enzymes, Lipid Profile and Antioxidant Enzymes in CCl4-Intoxicated Rats. Adv. Biol. Res. 2009, 3, 9–15.
- Nkosi, C.Z.; Opoku, A.R.; Terblanche, S.E. In Vitro antioxidative activity of pumpkin seed (Cucurbita pepo) protein isolate and its In Vivo effect on alanine transaminase and aspartate transaminase in acetaminophen-induced liver injury in low protein fed rats. Phytother. Res. 2006, 20, 780–783.
- Toma, I.; Victory, N.C.; Kabir, Y. The effect of aqueous leaf extract of fluted pumpkin on some hematological parameters and liver enzymes in 2,4-dinitrophenylhydrazine- induced anemic rats. Afr. J. Biochem. Res. 2015, 9, 95–98.
- Farid, H.E.; El-Sayed, M.S.; Abozid, M.M. Pumpkin and Sunflower Seeds Attenuate Hyperglycemia and Protect Liver in Alloxan-Induced Diabetic Rats. Res. J. Pharm. Biol. Chem. 2015, 6, 1269–1279.
- Chacko, K.R.; Reinus, J. Extrahepatic Complications of Nonalcoholic Fatty Liver Disease. Clin. Liver Dis. 2016, 20, 387–401.
- Ferramosca, A.; Zara, V. Modulation of Hepatic Steatosis by Dietary Fatty Acids. World J. Gastroenterol. WJG 2014, 20, 1746.
- Schwab, U.; Lauritzen, L.; Tholstrup, T.; Halldorsson, T.; Riserus, U.; Uusitupa, M.; Becker, W. Effect of the Amount and Type of Dietary Fat on Cardiometabolic Risk Factors and Risk of Developing Type 2 Diabetes, Cardiovascular Diseases, and Cancer: A Systematic Review. Food Nutr. Res. 2014, 58, 25145.
- Rezig, L.; Chouaibi, M.; Msaada, K.; Hamdi, S. Chemical Composition and Profile Characterisation of Pumpkin (Cucurbita maxima) Seed Oil. Ind. Crop. Prod. 2012, 37, 82–87.
- Herkeľ, R.; Gálik, B.; Arpášová, H.; Bíro, D.; Juráček, M.; Simko, M.; Rolinec, M. Fatty acid profile and nutritional composition of table eggs after supplementation by pumpkin and flaxseed oils. Acta Vet. Brno 2016, 85, 277–283.
- Hudečková, P.; Rusníková, L.; Straková, E.; Suchý, P.; Marada, P.; Macháček, M. The Effect of Linseed Oil Supplementation of the Diet on the Content of Fatty Acids in the Egg Yolk. Acta Vet. Brno 2012, 81, 159–162.
- AL-showayman, S.I.A. The Effect of Pumpkin Seed Feeding on The Serum Lipid Profile and C-Reactive Protein in Atherogenic Rats. King Saud Univ. Deansh. Grad. Stud. Dep. Biochem. Coll. Sci. King Saud Univ. 2010.
- Glew, R.; Glew, R.; Chuang, L.-T.; Huang, Y.-S.; Millson, M.; Constans, D.; VanderJagt, D. Amino Acid, Mineral and Fatty Acid Content of Pumpkin Seeds (Cucurbita spp.) and Cyperus esculentus Nuts in the Republic of Niger. Mater. Veg. 2006, 61, 49–54.
- Proboningsih, J.; Wirjatmadi, B.; Kuntoro, K.; Adriani, M. Expression of VCAM in Male Wistar Rats (Rattus Norvegicus) with Hypercholesterolemia Supplemented with Pumpkin Seeds (Cucurbita Moschata Duch) Extract. Health Notions 2018, 2, 648–654.
- Al-Okbi, S.Y.; Mohamed, D.A.; Kandil, E.; Abo-Zeid, M.A.; Mohammed, S.E.; Ahmed, E.K. Anti-Inflammatory Activity of Two Varieties of Pumpkin Seed Oil in an Adjuvant Arthritis Model in Rats. Grasas Aceites 2017, 68, 180.
- Lestari, B.; Meiyanto, E. A Review: The Emerging Nutraceutical Potential of Pumpkin Seeds. Indones. J. Cancer Chemoprevention 2018, 9, 92–101.
- Vijayalakshmi, S.; Kripa, K.G. Dietary Approaches in the Management of Rheumatoid Arthritis—A Review. Int. J. Res. Pharm. Sci. 2018, 9, 958–964.
- McVary, K.T.; Roehrborn, C.G.; Avins, A.L.; Barry, M.J.; Bruskewitz, R.C.; Donnell, R.F.; Foster, H.E.; Gonzalez, C.M.; Kaplan, S.A.; Penson, D.; et al. Update on AUA Guideline on the Management of Benign Prostatic Hyperplasia. J. Urol. 2011, 185, 1793–1803.
- Caro-Zapata, F.L.; Vásquez-Franco, A.; Correa-Galeano, É.D.; García-Valencia, J. Postoperative Infectious Complications after Open Prostatectomy and Transurethral Resection of the Prostate in Patients with Benign Prostatic Hyperplasia. Iatreia 2018, 31, 274–283.
- Edwards, R.; Shadiack, A. Do Pumpkin Seeds or Pumpkin Supplements Reduce Symptoms of BPH? Evid. Based Pract. 2018, 21, E14–E15.
- Vahlensieck, W.; Theurer, C.; Pfitzer, E.; Patz, B.; Banik, N.; Engelmann, U. Effects of Pumpkin Seed in Men with Lower Urinary Tract Symptoms Due to Benign Prostatic Hyperplasia in the One-Year, Randomized, Placebo-Controlled GRANU Study. Urol. Int. 2014, 94, 286–295.
- Xiong, X.M.; Yang, S.; Ming, K.; Xin-sheng, P.; Jue, C. Study on Extraction and Separation of Effective Composition of Pumpkin Polysaccharide and Its Glucatonic Effect. Chin. Tradit. Pat. Med. 2000, 22, 563–565.
- Ng, T.B.; Parkash, A.; Tso, W.W. Purification and Characterization of Moschins, Arginine–Glutamate-Rich Proteins with Translation-Inhibiting Activity from Brown Pumpkin (Cucurbita moschata) Seeds. Protein Expr. Purif. 2002, 26, 9–13.
- Park, S.-C.; Lee, J.R.; Kim, J.-Y.; Hwang, I.; Nah, J.-W.; Cheong, H.; Park, Y.; Hahm, K.-S. Pr-1, a Novel Antifungal Protein from Pumpkin Rinds. Biotechnol. Lett. 2009, 32, 125.
- Gill, N.; Bali, M. Isolation of Anti Ulcer Cucurbitane Type Triterpenoid from the Seeds of Cucurbita pepo. Res. J. Phytochem. 2011, 5, 70–79.
- Abdel-Azim, N.S.; Shams, K.; Shahat, A.A.A.; Missiry, M.; Ismail, S.I.; Hammouda, F.M. Egyptian Herbal Drug Industry: Challenges and Future Prospects. Res. J. Med. Plant 2011, 5, 136–144.
- Narayan, S.; Sasmal, D.; Mazumder, P.M. Evaluation of the Wound Healing Effect of Herbal Ointment Formulated with Salvia Splendens (Scarlet Sage). Int. J. Pharm. Pharm. Sci. 2011, 3, 195–199.
- Bahramsoltani, R.; Farzaei, M.H.; Abdolghaffari, A.H.; Rahimi, R.; Samadi, N.; Heidari, M.; Esfandyari, M.; Baeeri, M.; Hassanzadeh, G.; Abdollahi, M. Evaluation of Phytochemicals, Antioxidant and Burn Wound Healing Activities of Cucurbita Moschata Duchesne Fruit Peel. Iran. J. Basic Med. Sci. 2017, 20, 798.
- Inhorn, M.C. Middle Eastern Masculinities in the Age of New Reproductive Technologies: Male Infertility and Stigma in Egypt and Lebanon. Med. Anthropol. Q. 2004, 18, 162–182.
- Ejete-iroh, C.; Dada, A. Dietary Fluted Pumpkin (Telfairia occidentalis) Improves Reproductive Indices in Male African Catfish (Clarias gariepinus) Broodstock. J. Agric. Sci. 2019, 7, 228.
- Fawzy, E.I.; El Makawy, A.I.; El-Bamby, M.M.; Elhamalawy, H.O. Improved Effect of Pumpkin Seed Oil against the Bisphenol-A Adverse Effects in Male Mice. Toxicol. Rep. 2018, 5, 857–863.
- Abarikwu, S.O.; Mgbudom-Okah, C.J.; Onuah, C.L.; Ogunlaja, A. Fluted Pumpkin Seeds Protect against Busulfan-Induced Oxidative Stress and Testicular Injuries in Adult Mice. Drug Chem. Toxicol. 2019, 45, 1–11.
- Akang, E.N.; Oremosu, A.A.; Dosumu, O.O.; Noronha, C.C.; Okanlawon, A.O. The Effect of Fluted Pumpkin (Telferia occidentalis) Seed Oil (FPSO) on Testis and Semen Parameters. Agric. Biol. J. N. Am. 2010, 1, 697–703.
This entry is offline, you can click here to edit this entry!
